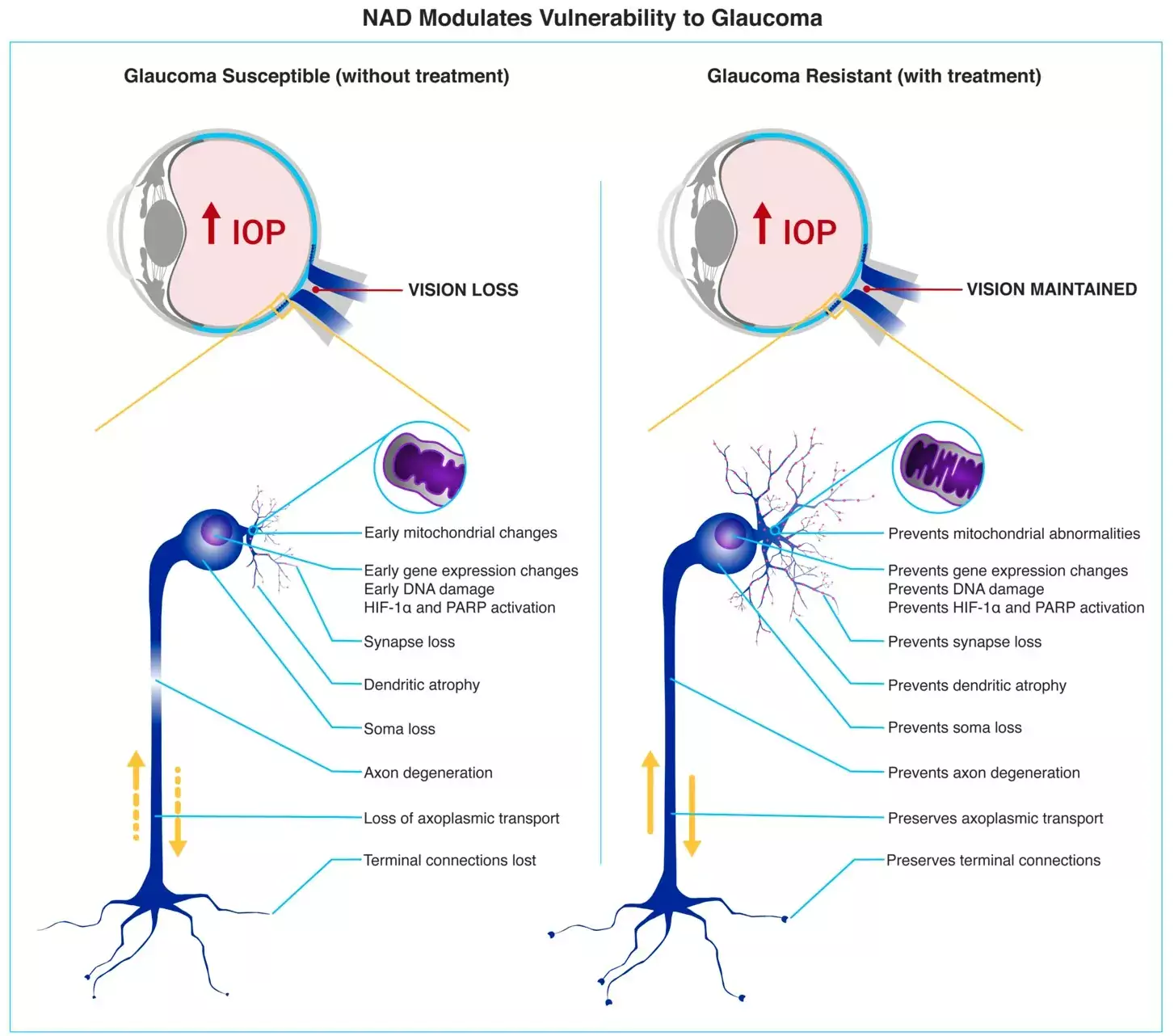


Glaukom (grön starr) är den vanligaste orsaken till irreversibel blindhet. Sjukdomen påverkar cirka 80 millioner världen över (cirka 100-200.000 i Sverige) och vad som orsakar glaukom är inte helt känt. Den enda bevisat verksamma behandlingen är att sänka ögontrycket men trots detta fortsätter många patienter att försämras.


Mitokondrier
Vi använder en mängd nya reportermöss, mitokondriella markörer och levande/vitala färgämnen för att avbilda och analysera mitokondrier med ultrahög upplösning i friska, sjuka och behandlade tillstånd.
Neuronal Metabolism
Vi använder riktade analyser, riktad och anrikad massa. spec. protokoll och högupplöst icke-riktad metabolomik för att identifiera nya sjukdomsvägar och kandidatbiomarkörer i glaukom.
Genterapier
Viral genterapi har varit framgångsrik vid en handfull sällsynta, monogena oftalmologiska sjukdomar. Vi utökar detta arbete för att utveckla genterapier som riktar sig mot vanliga neurodegenerativa vägar.
Neuroinflammation
Neuroinflammation i näthinnan och synnerven kan vara en kritisk patogen händelse vid tidig glaukom. Vår forskning utforskar mikrogliaaktivering i hela synsystemet vid glaukom.
 Foto: Rickard Kilström
Foto: Rickard KilströmPete Williams, docent och forskargruppsledare vid institutionen för klinisk neurovetenskap, har utsetts till professor i synvetenskap. Professuren finansieras av Ulla och Ingemar Dahlbergs stiftelse och har det fullständiga namnet "Ulla och Ingemar Dahlbergs professur i synvetenskap med inriktning mot okulär neurobiologi”. Pete Williams tillträdde som professor den 23 juni 2025. Professuren är inriktad på neuroprotektiv ögonforskning med syftet att hitta nya behandlingsformer för degenerativa ögonsjukdomar som glaukom.
 Foto: Pixabay,Mohamed Hassan
Foto: Pixabay,Mohamed HassanHjärnfonden stödjer varje år kvalificerad forskning om hjärnan och övriga nervsystemet samt sjukdomar, skador och funktionsnedsättningar i hela nervsystemet.Pete Williams, forskare vid Karolinska Institutet och S:t Eriks Ögonsjukhus, har tilldelats ett forskningsanslag från Hjärnfonden för sitt projekt: "NAD-metabolism som mål för neuroskydd vid åldersrelaterade oftalmiska och neurodegenerativa sjukdomar".
Anslaget, som uppgår till 2,4 miljoner kronor, kommer att finansiera forskningen under en treårsperiod.
 Foto: Johan Gunseus
Foto: Johan GunseusGlaukom – eller grön starr – är den vanligaste orsaken till permanent blindhet och drabbar 80 miljoner människor världen över. Nu har Pete Williams, forskare vid Karolinska Institutet och S:t Eriks Ögonsjukhus, tilldelats det prestigefyllda ERC Advanced Grant för sitt arbete med nya behandlingar mot sjukdomen. ERC Advanced Grant är en av de främsta utmärkelser som en forskare kan få.
Anslaget på 2,26 miljoner euro (cirka 25 miljoner kronor) över 5 år ger forskarlaget en kraftfull skjuts framåt.
 Foto: Sarvagna Mahakali
Foto: Sarvagna MahakaliI mycket hård konkurrens, med 10 000 sökande, har Sai Kocherlakota, ögonforskare inom glaukom vid Karolinska Institutet och S:t Eriks Ögonsjukhus tilldelats anslag inom EU:s främsta finansieringsprogram för forskning.
Sai Kocherlakota forskar inom ögonsjukdomen glaukom, och tilldelas anslaget för att utveckla nanopartikelmedierade behandlingar som riktar sig mot nervcellernas ämnesomsättning.
 Foto: Flora Hui
Foto: Flora HuiPete Williams, forskare vid Karolinska Institutet och S:t Eriks Ögonsjukhus, har tilldelats ett forskningsanslag från den amerikanska stiftelsen The Glaucoma Foundation för att utveckla en behandling för glaukom och andra ögonsjukdomar. Med anslaget på USD 75 000 (SEK 840 000) vill stiftelsen uppmärksamma banbrytande, innovativ glaukomforskning. Det är andra gången Pete Williams får anslaget.
 Foto: Kommunikationsavdelningen S:t Eriks Ögonsjukhus
Foto: Kommunikationsavdelningen S:t Eriks ÖgonsjukhusTre forskare vid S:t Eriks Ögonsjukhus och Karolinska Institutet, får totalt 1, 5 miljoner kronor i anslag från Stiftelsen Kronprinsessan Margaretas Arbetsnämnd för synskadade och Synskadades Vänner i Gävleborg för forskning kring behandling av glaukom.
 Foto: Stefan Zimmerman
Foto: Stefan ZimmermanPete Williams är en av få i Sverige som forskar på glaukom eller grön starr som det också kallas. Målet är en effektivare behandling. Ett läkemedel som kan förhindra den nedbrytande processen i ögats nervceller.
 Foto: Johan Gunseus
Foto: Johan GunseusPete Williams, forskargruppsledare och lektor vid Karolinska Institutet och S:t Eriks Ögonsjukhus, har beviljats 600 000 kronor i anslag från stiftelsen Promobilia för sitt projekt med titeln Dubbel inriktning av neuroprotektion och neuroregeneration i synnerven. Projektet kommer att pågå i två år.
På den engelska sidan kan ni läsa mer om aktuell och pågående forskning och mer om Pete William´s forskargrupp.