Our research
Our goal is to understand critical cancer pathways so that we can suggest better cancer treatments and preventive approaches, as well as biomarkers, to be developed for clinical use.
Non-coding RNA molecules are RNAs that do not encode for proteins. The family includes microRNAs which regulate translation of target mRNAs and long non-coding RNAs (lncRNAs) which can have active functions such as 3D-RNA molecules. Both types can function as biomarkers or therapeutic targets. We study how non-coding RNAs are impacted by cell differentiation, hormone signalling and cancer, and their corresponding functions.
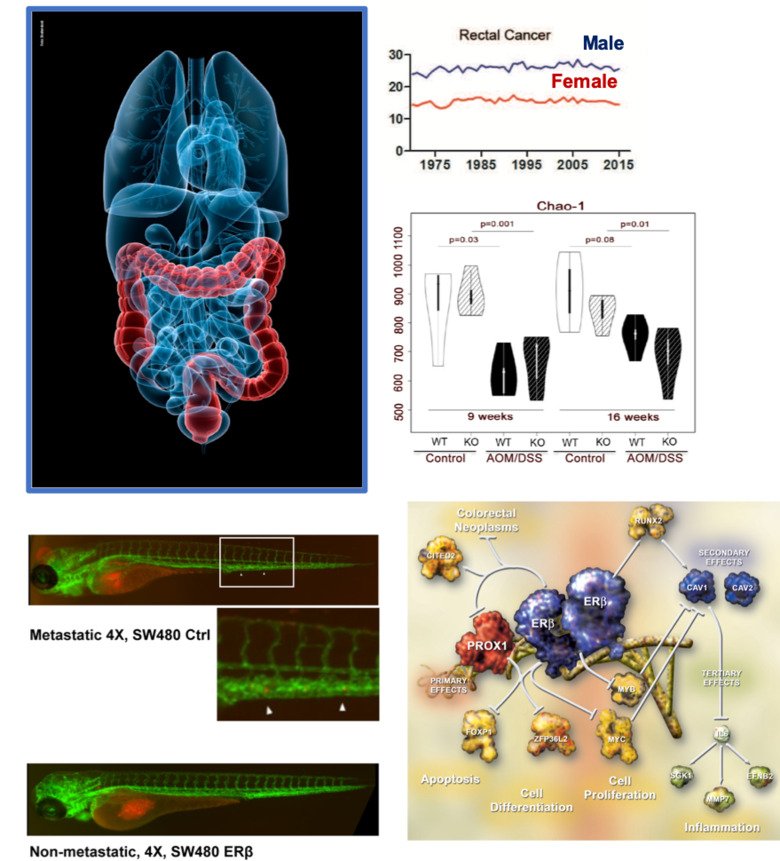
Oestrogen signalling and sex differences in cancer
Some cancers (such as colorectal and liver) are more common in men, and others (breast and thyroid) are more common in women. We explore how the hormone oestrogen influences this and, for example, increases the growth of breast cancer while protecting against colorectal cancer.
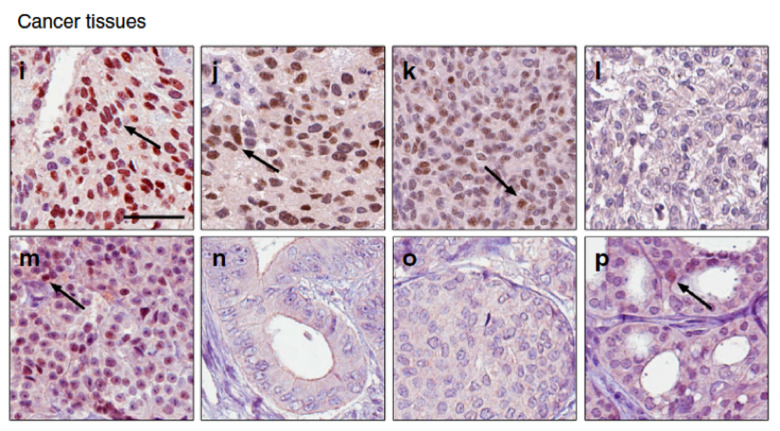
The effect of oestrogen is mediated by three oestrogen receptors: ERα, ERβ and GPER1. These are expressed to various extent in different tissues in both women and men. ERα and ERβ are ligand-activated nuclear receptors and are excellent therapeutic targets. GPER1 is a G protein-coupled transmembrane receptor with promising therapeutic potential. Using technologies like RNA-Seq, ChIP-Seq, microbiota analysis, and proteomics, along with cell models, tissue-specific knockouts and clinical samples, we explore how these three receptors, and their ligands such as dietary oestrogens or endocrine disruptors, impact cancer. We are interested in understanding how this contributes to sex differences in cancer incidences and outcomes, as well as the intricate connection with diet/obesity, inflammation and carcinogenesis. Our goal is to contribute to better biomarkers, therapeutics and preventive approaches.
Research Networks
- Pan-Gynecologic Cancers Analysis Working Group, The Cancer Genome Atlas
- VR Research environment Interdisciplinary research (QuantumSense 2018-2025)
- American Association for Cancer Research (AACR)
Prizes/Awards
- Career Development Award (VINNOVA/ Marie-Curie Actions GROWTH 291795, Cecilia Williams, 2014-2018)
- National Cancer Institute / NIH USA (R01 CA172437, 2013-2019)
