Our research
Many DNA-damaging anti-cancer drugs cause replication-associated DNA damage that kills cancer cells. This is an effective way of treating cancer, but the problem is that also normal cells are damaged. Our strategy is to exploit the high level of DNA damage and dysfunctional redox status in cancer cells and prevent the repair of these lesions and even cause more oxidative lesions in the cancer cells. Using DNA repair inhibitors, we can selectively introduce toxic DNA damage to cancer cells.
Our focus is on the most common DNA lesions, base lesions, that are substrates for base excision repair (BER), a repair pathway discovered by Tomas Lindahl for which he got the Nobel Prize, and involves several glycosylases, AP-endonucleases, Poly (ADP-ribose) Polymerase (PARP) and ligases. Earlier, the Helleday group pioneered the synthetic lethal concept for the treatment of cancer (Bryant et al., Nature 2005), which exploits that a mutated cancer requires a backup pathway for survival, not normally required for normal cells to survive. Using this concept, we demonstrated effective treatment of BRCA2 defective tumors with PARP inhibitors, which did not cause the side effects associated with chemotherapy treatments.
We are currently building on this strategy and expanding into targeting novel DNA repair enzymes. We demonstrated how targeting MTH1 can be used to target cancer in general without causing toxicity to non-transformed cells (Gad et al, Nature 2014; Huber et al., Nature 2014) and we have developed the MTH1 inhibitor (MTH1i) karonudib (Karolinska NUDT1 inhibitor) (Warpman Berglund et al., Ann Oncol 2016) and progressed it through GMP manufacturing, formulation, GLP safety assessment, IMPD, Medical Product Agency and ethical approvals. Presently two clinical Phase 1 trials (one in patients with advanced solid malignancies and one in hematological cancers) with karonudib are ongoing at Karolinska University Hospital. Pharmacology and biology are often more complex than originally thought, and we continue to deepen our understanding of the mechanism of action of our MTH1i and the role of MTH1 in cancer pathology.
By targeting the OGG1 glycosylase, we show how this can be used to suppress the onset of inflammatory and immune processes in for instance sepsis (Visnes et al., Science 2018).
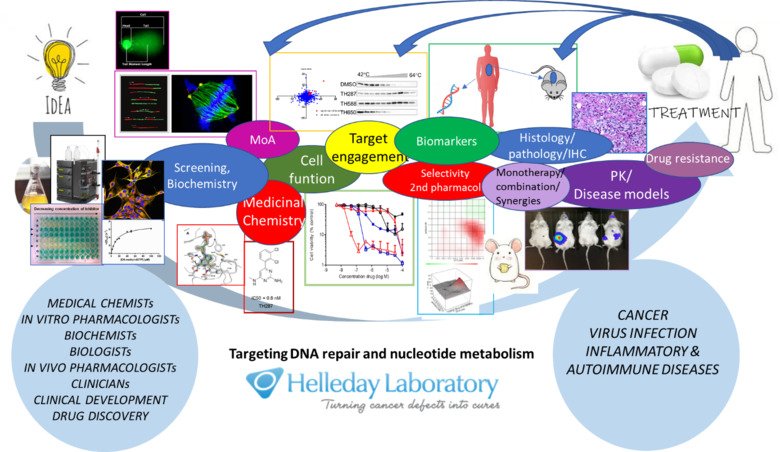
Overreaching goals:
- Mechanistic understanding of DNA repair and nucleotide synthesis proteins with a focus on small base lesions.
- Purification of proteins, identification of functions and novel inhibitors with co-crystal information and develop pharmacological drugs to progress into treatments.
- Develop targeted therapy in clinical trials
- Develop ex vivo screening as companion diagnostic to treatments.
Current projects/targets
1) MTH1 – We have identified novel functions of MTH1 in mitosis and have made potent MTH1 inhibitors one of which, karonudib, is currently evaluated in clinical phase I trials. We are investigating combination treatments, biomarkers, potential resistance mechanisms as well as MTH1 potential role in inflammation/immune processes.
2) OGG1 –We developed first-in-class OGG1 inhibitors that we are progressing in lead optimization for treatments of inflammation and to further understand the detailed role in DNA repair. We have also identified OGG1 enhancers and are evaluating their potential in various disease models. We are also exploring OGG1 as a potential treatment for cancer.
3) MTHFD2 – We are exploring novel biological roles of MTHFD2 and we have generated potent inhibitors to this target that show anti-cancer properties. We are fast-tracking MTHFD2 inhibitors into the clinic.
4) NUDT22 – is an understudied enzyme and we have identified its biochemical and biological function. We have generated potent NUDT22 inhibitors to be tested for treatment applications and to further expand our understanding of the role of the enzyme.
5) Base excision repair proteins – we are purifying a number of DNA glycosylases and other base excision repair proteins and are probing their biochemical roles to develop inhibitors, which may be applied for the treatment of human diseases.
6) Ex vivo screening – we are collaborating with Päivi Östling and Olli Kallionemi for ex vivo screening and precision cancer medicine in AML as well as collaborating with the University of Sheffield.
7) Virus- we have found that some of our inhibitors kill viruses. We are further investigating the underlying mechanism of actions and potential effects as treatments.
The Helleday group consists of four teams; Biochemistry and In Vitro pharmacology (Team Leader Dr. Ann-Sofie Jemth), In vivo pharmacology (Team Leader Dr. Kumar Sanjiv), Basic Science (Team Leader Dr. Oliver Mortusewicz) and Medicinal Chemistry (Team Leader Dr. Olov Wallner). The research teams are focused on DNA repair and nucleotide metabolism.