Our research
Atherosclerotic cardiovascular disease including coronary artery disease (CAD) and myocardial infarction remains leading cause of morbidity and mortality. Despite recent progress, the mechanisms remain unclear. Occurrence of endothelial dysfunction primarily characterized by reduced availability of nitric oxide (NO) increased oxidative stress is an early factor during development of CAD and tissue injury during ischemia-reperfusion.
Our research is focused on the identification of disease mechanisms behind endothelial dysfunction, and development of novel therapeutic strategies to improve endothelial function in coronary artery disease (CAD) and limit the extent of myocardial infarction.
This is of particular importance in diabetes and hyperlipidemia which considerably increases the risk for CAD. We have identified key regulators of NO including the enzyme arginase that is upregulated in CAD and diabetes and reciprocally regulates NO formation and oxidative stress. Targeting arginase improves endothelial function and protects against myocardial ischemia-reperfusion injury both by increasing NO production and reducing the formation of reactive oxygen species (ROS).
Our recent data suggest an intriguing interaction between red blood cells (RBCs) and the endothelium in the development of cardiovascular injury in type 2 diabetes. This effect appears to be driven by increased arginase activity and ROS formation in RBCs in type 2 diabetes.
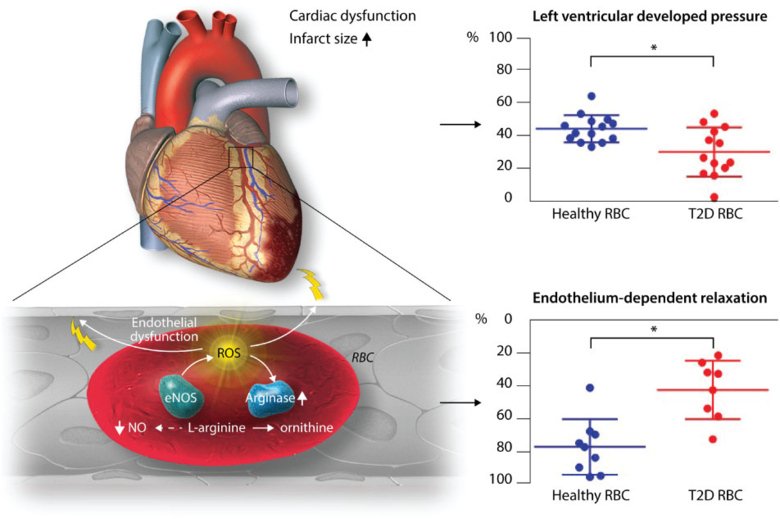
Ongoing projects
In ongoing projects we investigate:
- the effect of RBCs from different patient groups with risk factors for and developed atherosclerotic cardiovascular disease on cardiac and endothelial dysfunction
- the signaling mechanisms underlying cardiac and vascular injury caused by RBC from patients with and animal models of type 2 diabetes, hypercholesterolemia and myocardial infarction
- the effect of pharmacological interventions on RBC and endothelial function in these patient groups
- the impact of co-morbidities on cardioprotective effects of pharmacological and ischemic conditioning on acute myocardial infarction
Research methods and models
We perform translational studies ranging from molecular to clinical studies. Molecular studies are focused on understanding of the role of NO signaling pathway in RBCs, endothelial cells and the vascular wall. Additional approaches are focused on signaling by non-coding RNA. Experimental models include isolated heart preparations, isolated vessels, cell culture and in vivo models of myocardial infarction. We also perform investigations of endothelial function in humans, which include early interventional studies exploring novel targets in patients with CAD and risk factors. Finally, the group performs randomized clinical studies with functional and clinical endpoints.
Significance
The project will provide novel insights into molecular mechanisms driving development of CAD and myocardial ischemia-reperfusion injury. Targets identified in experimental studies are tested in early clinical interventional studies that will provide the basis for novel therapeutic strategies.
