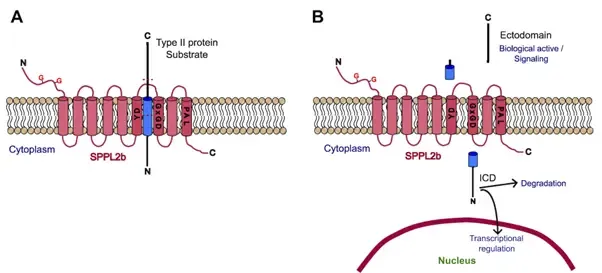
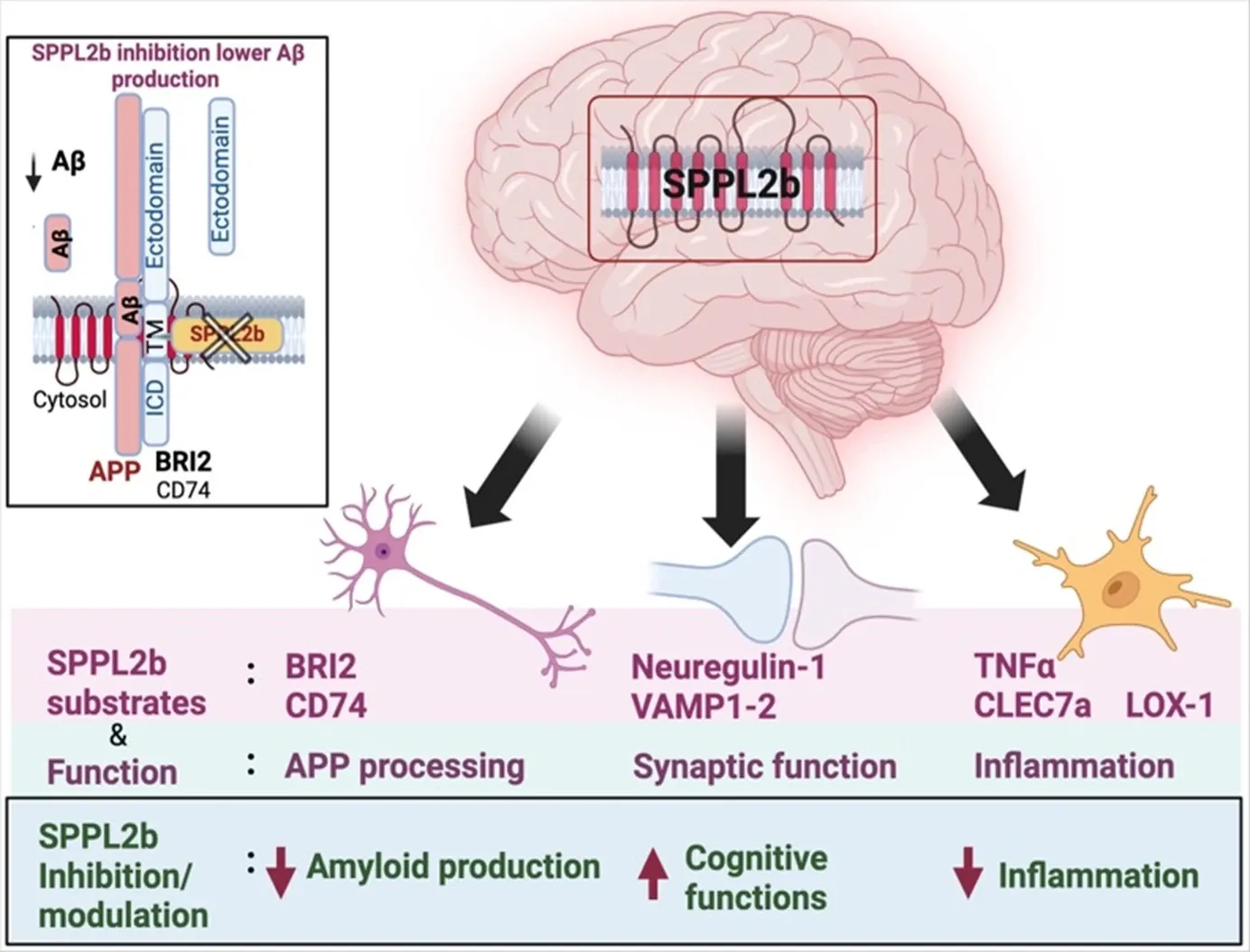
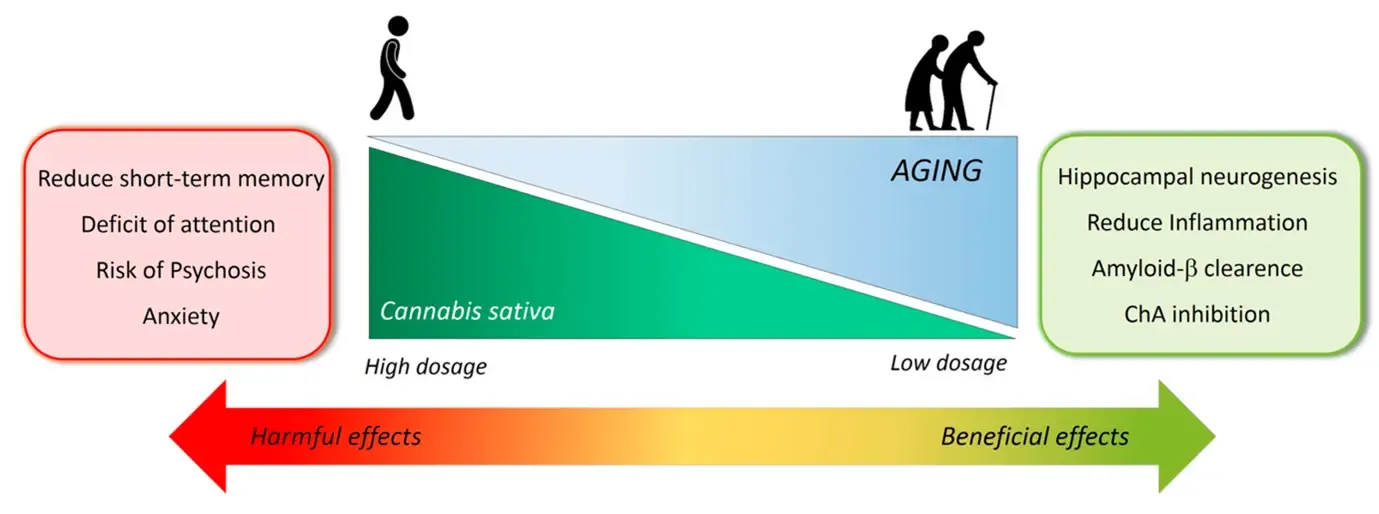
Research focus
Alzheimer’s disease (AD) is characterized by changes in protein homeostasis which leads to accumulation and aggregation of proteins that are toxic to cells in the brain. In AD, these include amyloid beta peptide (Aβ) and tau protein. Autophagy is a master regulator of proteostasis which maintain and adjust the proteome depending on the environment of the cell. This ensures that misfolded and potentially toxic proteins are degraded and at the same time refill the pool of amino acids that will serve as source for the for synthesis of novel proteins. Autophagy is malfunctioning in the Alzheimer’s disease brain and may therefore contribute to the disease progression.
We are analyzing the mechanism of autophagy in the metabolism of Aβ using state of the art AD mouse models. Using genetic tools, we inhibit autophagy in different neuronal cells. Intriguingly, we have found that when autophagy is deleted in the nerve cells, the extracellular Aβ plaques decrease and Aβ instead accumulates intracellularly. This activates neurodegenerative processes which could be linked to the neurodegeneration taking place in the AD brain. This neurodegeneration is currently being investigated by different genetic and omics approaches.
Aβ levels are increased in both familiar and sporadic AD. In familiar AD, genetic mutations cause the increase in Aβ while in sporadic AD a decreased degradation of Aβ may explain the augmented levels. Neprilysin is the major Aβ-degrading enzyme which is controlled by somatostatin receptor (SSTR) signaling. Our aim is to identify blood-brain-barrier-permeable SSTR agonists that activates neprilysin in high through put screens to lower Ab levels.