Our research
The group's research aims to understand how the tissue microenvironment influences the development and function of myeloid immune cells in homeostasis, infection and chronic inflammatory conditions. In particular, the focus is on the ability of tissue-specific cells, especially fibroblasts, to shape the function of myeloid cells.
We use the latest technology in high-plex spatial protein profiling and take advantage of our recent advances in the development of organotypic tissues with immune cells. These immunocompetent organotypic tissues recreate important properties of specific tissues (lung, skin, tonsil and gingiva), which have proven to be robust tools for modelling homeostasis, inflammation and infection. The goal is to demonstrate new mechanisms of tissue homeostasis and inflammation, as well as the functional relationship of fibroblasts with myeloid immune cells in infection and inflammatory disease, thereby paving the way for new therapeutic approaches targeting fibroblasts.
Studies on human fibroblasts´s and myeloid immune cells
We investigate the heterogeneity and functionality of fibroblasts across various tissues, analysing human organ donor tissue and tissue from patients suffering from bacterial infections or chronic inflammatory conditions. Fibroblast subsets and states are investigated through analyses of differentially expressed genes at a single-cell level.
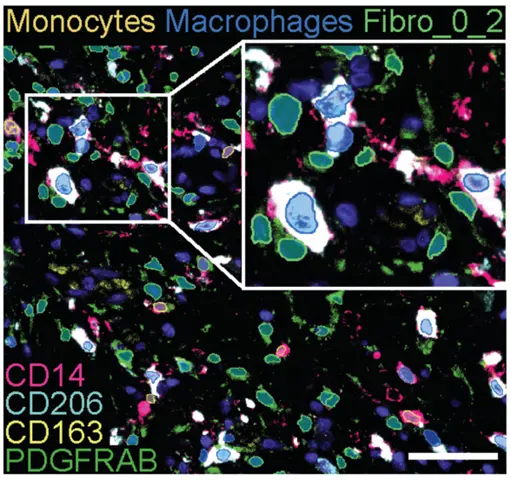
Fibroblast heterogeneity revealed at a transcriptional level is validated at protein and cellular levels using multi-parameter flow cytometry and high-plex spatial imaging analyses. Fibroblast and myeloid cell relationships are studies using organotypic and ex vivo tissue models in which their phenotypes and functions will be investigated with multiparameter imaging and flow cytometry analyses and deep expression profiling in combination with myeloid cell-T cell stimulation assays.
Technologies
For our research we employ specialized cultures of tissue (organotypic) as well as ex vivo explant models together with advanced technologies including confocal microscopy (Nikon A1R), high-plex spatial protein profiling (MACSimatm), flow cytometers (BD Symphony A3 and A5), cell sorters (Sony MA900), single-cell sequencing (10X genomics), and multiplex protein analysis (Luminex)
Collaborations
Our group closely collaborate with other research groups at Center for Infectious Medicine, as well as several national and international researchers
- Kristoffer Strålin, Karolinska University Hospital, Karolinska Institutet, Sweden
- Olav Rooijackers, Karolisnak Institutet, Sweden
- Teresa Frisan, Umeå University, Sweden
- Fredric Carlsson, Lund University, Sweden
- Edoardo Saccenti, Wageningen University, Netherlands
- Andrew Steer, Murdoch Children´s Research Institute, Melbourne, Australia
- Hanna Frost, Murdoch Children´s Research Institute, Melbourne, Australia
- Joshua Osowicki, Murdoch Children´s Research Institute, Melbourne, Australia
Open positions
We are constantly looking for talented potential co-workers. If you are interested in doing research within our group, as a degree project or as a researcher, please contact the Group leader: mattias.svensson@ki.se.

