
- >
- >
- Reproductive endocrinology and metabolism – Elisabet Stener-Victorin's research group
- Tissue engineering – Magdalena Fossum team
- Inflammation and metabolism – Nicolas Pillon's team
- Neurobiology of Sensory Systems – François Lallemend group
- Experimental Traumatology Research Unit
- Genetic Modification of NK cells for Optimized Functions against Cancer – Arnika Wagner team
- NK cells in the development of adaptive immune responses – Benedict Chambers team
- Molecular pain research – Camilla Svensson's research group
- Brain Connectomics – Joana Pereira's research group
- Genomic Science and RNA Biology – Vicent Pelechano group
- Immunotherapy – Robert Harris' research group
- Infectious diseases "Venhälsan" – Jaran Eriksen's research group
- Musculoskeletal disorders from a biopsychosocial perspective – Wim Grooten's research group
- Hjerling-Leffler group
- Respiratory and invasive infections and microbial pathogenesis – Birgitta Henriques-Normark Laboratory
- Multimodal Brain Imaging – Daniel Lundqvist's research group
- Obsessive - Compulsive and Related Disorders Across the Lifespan – David Mataix-Cols research group
- With a focus on proteins for better cancer treatments – Pär Nordlund's Group
- Precision cancer medicine and precision radiotherapy in lung cancer - from molecular mechanisms/biomarkers to clinical trials – Kristina Viktorsson's team
- Cancer Bioinformatics – Nick Tobin's Team
- Lipoproteins and the Immune System – Research group Stephen Malin
- Equity and Health Policy (EHP) – Ann Liljas' research group
- Effects of hypoxia in physiological and pathological contexts, Hypoxia Inducible Factors (HIF) – Randall Johnson's Group
- Chemistry II – Jesper Z. Haeggström group
- Genomic analysis of parasites and viruses, metagenomic sequencing – Björn Andersson's Group
- Translational control of cancer – Ola Larsson's Group
- Gastrointestinal Surgery KI SÖS – Gabriel Sandblom's research group
- Understanding the mechanisms behind hantavirus-mediated pathogenesis – Jonas Klingström group
- The Gynaecological research team – Elisabeth Epstein
- Understanding how life develops – Emma Andersson's Group
- Statistical methods in epidemiology – Paul Dickman's research group
- Neurons and Neural Networks – Ole Kiehn group
- Research groups in developmental biology
- Behavioral, Resilience and Digital Health in Pain – Rikard Wicksell’s research group
- Research groups in neurology
- Research groups in epidemiology, global public health and social medicine
- Nordic Brain Network – Miia Kivipelto's research group
- Resilience and mental health – Serhiy Dekhtyar group
- Forensic behavioral and neuroscience – Katarina Howner's Research Group
- Immune Engineering – Team Leo Hanke
- Chemical and physical exposure in the work environment and health – Jenny Selander's research group
- Mechanobiology of cardiac regeneration – Elif Eroglu's Group
- Tumors of the female genital organs – Miriam Mints' research group
- Psychoneuroimmunology – Mats Lekander's research group
- Research groups in odontology
- Neuroimmunovascular biology – Harald Lund's team
- Improved synthetic DNA structures by evolutionary selection – Erik Benson Group
- Lifestyle and Kidney Cancer – Stephanie Bonn Team
- Neuroplasticity and Regeneration – Konstantinos Ampatzis group
- Paediatric cell and molecular biology – Hjalmar Brismar's research group
- Quantitative Biology of the Nucleus – Magda Bienko Group
- Cardiometabolic factors, brain aging, and dementia care – Weili Xu group
- Cognitive Neuroscience of Body and Self – Henrik Ehrsson Group
- Surgical Care Science – Pernilla Lagergren's research group
- Clinical and translational studies on viral hepatitis – Soo Aleman group
- Cell and Gene Therapy – Evren Alici group
- Personalized Medicine and Drug Development – Lauschke-lab
- Electrophysiological neuropharmacology – Göran Engberg's research group
- Mechanisms of Pain and Treatment – Eva Kosek's research group
- Functional (Epi)genomics of Neuroinflammatory Diseases – Maja Jagodic's research group
- Molecular basis of gene regulation of diseases – Carsten Daub's research group
- Health risk assessment methodology – Anna Beronius' group
- Medical genetics – Catharina Larsson's Group
- Translational Genetics of Neurodegenerative disease – Caroline Graff's research group
- Development, transcription factors, neurons, dopamine – Thomas Perlmann's Group
- Clinical and translational research within lupus and autoimmunity – Team Ioannis Parodis
- Cell cycle, mitotic entry, and DNA-damage checkpoints – Arne Lindqvist's Group
- Breast Surgery – Irma Fredriksson's research group
- Laura Orellana's Group
- Research group Ali Mirazimi
- Early drug discovery research in chronic inflammatory diseases – Research group Michael Sundström
- Integrative Physiology – Juleen Zierath's research group
- Simon Elsässer group
- Clinical Neuroimmunology and Immunomodulation – Fredrik Piehl's research group
- Understanding mechanisms of vaccination – Research group Karin Loré
- Systems regenerative neurobiology – Enric Llorens Group
- Clinicial Cancer Genomics – Johan Lindberg's research group
- Jeanette Hellgren Kotaleski group
- Brain control of food intake and body weight – Björn Meister group
- Research groups in endocrinology and diabetes
- Research groups in gastroenterology and hepatology
- Research groups in neurosciences
- Research groups in respiratory medicine and allergy
- Caring in Community Care – Zarina Nahar Kabir's research group
- Molecular mapping of the nervous system in health and disease – Jan Mulder group
- Child and adolescent psychiatry – Jens Högström's research group
- Genetic mechanisms of ageing – Maria Eriksson's research group
- Digital Psychiatry – Philip Lindner's research group
- Synaesthesia, autism and perception – Janina Neufeld's team
- Vascular complications in cardiometabolic disease – Team Zhichao Zhou
- Lars Karlsson team
- Sepsis and microcirculation – Sara Tehrani's research group
- Translational Clinical Orthopaedics – Michael Axenhus Research Group
- Antigen-specific T cell repertoires – Cassotta lab
- Translational Molecular Imaging – Nordberg Lab
- Neuroplasticity – Dagliyan group
- Developmental and Translational Neurobiology – Cristiana Cruceanu's research group
- Microbiota–gut–brain axis and neurodevelopment – Rochellys Diaz Heijtz group
- Thoracic Surgery – Anders Franco-Cereceda's research group
- Electrophysiology – Mats Jensen-Urstad group
- Role of T cells in human host defense – Johan Sandberg group
- Germ cell biology and developmental programming in epigenetic inheritance of diseases – Qiaolin Deng's research group
- Unit for Bioentrepreneurship – Hanna Jansson's group
- Health informatics – Sabine Koch's group
- Cancer, rapid ageing and nutrition – Martin Bergö's research group
- Clinical radiation therapy – Åsa Carlsson Tedgren's Group
- TRANslational Theranostics Group – Thuy Tran
- Pathogenic pathways in Alzheimer Disease – Lars Tjernberg's research group
- Hematological and solid tumors – Research group Xu Dawei
- Developmental Neurogenomics – Michael Ratz's Group
- Notch signaling, ES cells, myogenic and vascular progenit breast cancer – Urban Lendahl's Group
- Particle toxicology – Hanna Karlsson's group
- Mitochondrial dysfunction in Alzheimer Disease – Maria Ankarcrona's research group
- Molecular pathology of the lung and pleura – Katalin Dobra's Group
- Late effects and cancer survivorship after diagnosis and treatment of aggressive lymphoma – Team Sandra Eloranta
- Research Group Anna Nopp
- Coding and non-coding RNAs in cancer – Per Hydbring's Team
- Research group Mikael Björnstedt
- Sister chromatid cohesion in DNA damage responses, DSB repair, Genome Integrity, Gene regulation – Lena Ström's Group
- Inborn Errors of Endocrinology and Metabolism – Anna Wedell's research group
- Functional Precision Medicine – Brinton Seashore-Ludlow's Team
- Understand skin using modern biology – Maria Kasper's Group
- Integrative Epidemiology – Fang Fang's research group
- Molecular Biometry – Roman Zubarev group
- Idiopathic pulmonary fibrosis (IPF) – Research group Magnus Sköld
- Molecular Neuroscience – Carlos Ibáñez group
- Research groups in psychology
- Research groups in forensic science
- Research groups in medical genetics and genomics
- Research groups in nursing
- Research groups in rheumatology and autoimmunity
- Genetic epidemiology of prostate and testicular cancer – Fredrik Wiklund's research group
- Systems biology of aging – Juulia Jylhävä's research group
- Aging – basic mechanisms and interventions – Christian Riedel's research group
- Embryonal, foetal and brain development – Juha Kere's research group
- Research groups in occupational therapy
- Growth and Cartilage Biology – Ola Nilsson's research group
- Infections and immunity in children with cancer – Anna Nilsson's research group
- Oxygenation, early resuscitation in trauma and drug treatment in cardiac arrest – Team Malin Jonsson Fagerlund
- Prehospital Emergency Care – Veronica Vicente's Research Group
- The brain after surgery and trauma - neuroinflammation, cognitive impairment and dementia – Lars I Eriksson research group
- Personalized diet and medications for cognition – Garcia-Ptacek group
- Arthroplasty – Olof Sköldenberg’s research group
- Anna Undeman Asarnoj team
- Visual Neuroscience – Rune Brautaset's research group
- Chemicals and female fertility – Pauliina Damdimopoulou Research Group
- Molecular and cellular exercise physiology – The Ruas Lab
- Neural circuits of cognition – Marie Carlén group
- Colorectal Surgery – Anna Martling and Caroline Nordenvall's research group
- Myeloma group – Evren Alici's research group
- Medicine and history of science – Eva Åhrén's group
- Cardiometabolic diseases – Ping Chen research group
- Anders Mutvei research group
- Ocular Oncology and Pathology – Gustav Stålhammar's research group
- Clinical Neurophysiology – Charith Cooray's research group
- Regulation of Gene Expression during Viral Infection – Gerald McInerney Group
- Social and affective learning and decision-making – Andreas Olsson's research group
- Translational Breast Cancer Research: Long-Term Risk and Endocrine Treatment Benefit – Linda Lindström's Group
- Notch3 and the cerebral small vessel disease CADASIL – from molecular mechanisms to treatment strategies – Helena Karlström's research group
- Dementia and Multimorbidity – Dorota Religa's research group
- Diagnostic Radiology – Lennart Nedar's research group
- Cognitive Neuropsychiatry – Predrag Petrovic's research group
- Chemical carcinogenesis – Ulla Stenius' group
- Metal toxicology – Maria Kippler's research group
- Cellular Stress in Health and Disease – Federico Pietrocola's group
- Autophagy and cardiovascular disease – Team Ewa Ehrenborg
- Research group Birgitta Sander & Birger Christensson
- Innate Immune Regulation – Quirin Hammer team
- Genetics of tumor predisposition and progression in breast cancer – Svetlana Bajalica Lagercrantz's Team
- Implementation and Quality (IMPAQT) – Claudia Hanson's research group
- Structural and Biophysical Immunology – Research group Adnane Achour
- Ulrika Marklund group
- Sustainable work & occupational safety and health – Emma Brulin's research group
- Health Systems Leadership, Management, and Safety – Carl Savage's group
- Cell and Molecular Immunology - Research group Cecilia Söderberg Naucler
- Brain Circuits – Konstantinos Meletis group
- Petter Ljungman's research group- Health effects of the Ambient Environment
- Neuropsychology of music – Fredrik Ullén group
- Research groups in geriatrics and gerontology
- Research groups in nutrition and dietetics
- Research groups in sports and fitness sciences
- Prostate Cancer – Henrik Grönberg's research group
- Methods of health promotion in the workplace – Lydia Kwak's research group
- Autism and perinatal epidemiology – Sven Sandin research group
- Wengström's research group
- Modelling and simulation with applications to cancer epidemiology and health economics – Mark Clements' research group
- Health Technology Assessment – Monica Hultcrantz’s group
- Pediatric anesthesiology Stockholm – Per-Arne Lönnqvist's research group
- Biophysics – Erdinc Sezgin's Lab
- War, crisis, and security studies
- Systems Medicine – Johan Björkegren research group
- Trauma – Max Gordon's research group
- Perpetration Prevention – Rahm and Joleby's research group
- SOLIID - Sustainable Organizational Learning, Innovation, Improvement and Development in Health and Social services – Monica Nyström's group
- Social Gerontology – Carin Lennartsson group
- Genetic and molecular basis of nervous system disorders – Andrea Carmine Belin group
- Chronobiology – Petrus lab
- Leukemic stem cells – Petter Woll group
- Nanomedicine and Spatial Biology – Teixeira Lab
- Global Disaster Medicine - Health Needs and Response – Johan von Schreeb's research group
- Translational cardiac and skeletal muscle physiology – Daniel Andersson's research group
- Neuroradiology – Staffan Holmin's research group
- Tumour genomics – Lauri Aaltonen's research group
- Neurobiology of Motor Actions – Abdel El Manira group
- Neuroinflammation in Alzheimer disease – Marianne Schultzberg research group
- Palliative Medicin – Linda Björkhem-Bergman's research group
- Nucleotide metabolism and molecular pharmacology – Sean Rudd's Group
- Cardiovascular Inflammation – Research group Peder Olofsson
- Mesenchymal stem cells – Katarina Le Blanc group
- Luigi De Petris' Team
- Autoimmunity and cancer – Research group Marie Wahren-Herlenius
- Atherothrombosis research – Team Nailin Li
- Precision cancer medicine – Olli Kallioniemi's group
- Research group Stephan Mielke
- Genetics of autoimmune diseases – Team Leonid Padyukov
- Musculoskeletal disorders; planetary health & biopsychosocial approaches – Nina Brodin's research group
- Vascular Functions in Metabolic and CNS Diseases – Ulf Eriksson group
- Sarcoma Genomics – Felix Haglund de Flon's Group
- Team Helene Alexanderson
- Injury and Repair in the Immature Brain – Blomgren group
- Statistical methods for cancer patient survival – Therese Andersson's research group
- Individual differences in cognition – Agneta Herlitz’ research group
- Research groups in health economy, healthcare management and policy
- Research groups in reproductive medicine and gynaecology
- Research groups in structural biology
- Research groups in artificial intelligence
- Work environment, health and productivity – Christina Björklund's research group
- Predictive medicine – Mattias Rantalainen's research group
- Epigenetic regulation of leukemia and normal blood development – Andreas Lennartsson's research group
- Epigenetic mechanisms underlying metabolic-inflammatory diseases – Eckardt Treuter's research group
- The IMPACT research group – Marie Löf
- Stockholm Center for Health Economics (StoCHE) – Emelie Heintz's team
- Towards personalized physiology in Intensive Care – Team Johan Mårtensson
- Ninib Baryawno research group
- Neurohormonal Basis of Physiological and Maladaptive Expressions of Survival Behaviors – Stefanos Stagkourakis group
- Breast cancer precision medicine – Marike Gabrielson's research group
- D-WHIN: Danderyd Wrist and Hand INitiative – Research group
- Research group Kilian Eyerich
- Global infections – Research group Anna Färnert
- Translational Microbiome Research and Pandemic Preparedness – Lars Engstrand group
- EcoMind and biological pathways in cognitive aging – Debora Rizzuto group
- Circuits controlling Action and their Evolution – Sten Grillner group
- Mikael Rydén & Niklas Mejhert lab
- Understand Fundamental Immune Mechanisms to Develop New Treatments for Lung Diseases – Tim Willinger group
- Clinical Brain Imaging Methods – Anna Falk Delgado's research group
- Hyperbaric medicine – Peter Lindholm's research group
- Eye movements and vision – Tony Pansell's research group
- Lung toxicology – Lena Palmberg's research group
- The NeuroCardioMetabol Group – Thomas Nyström & Cesare Patrone
- Environmental and genetic factors influence on neurodevelopment – Sandra Ceccatelli group
- Skin wound healing – Research group Ning Xu Landén
- From development to disease: decoding stem cell programs for CNS repair – Erik Sundström's and Xiaofei Li's research group – Erik Sundström-Xiaofei Li group
- Neurodegenerative diseases and Aging – Daniel Ferreira Research Group
- Deep learning models of cancer mechanisms – Avlant Nilsson's Group
- Clinical Physiology – Marcus Carlsson's research group
- Molly Stevens group
- Molecular Mechanisms of Viral Oncogenesis – Maria G. Masucci's Group
- Björn Reinius group
- Quantitative principles in tumour biology – Jean Hausser's Group
- Bone biology – Sara Windahl Research group
- Research group Volkan Özenci
- Genetics and mechanisms of metals – Karin Broberg's research group
- Immunodeficiency Diseases – Lisa Westerberg Group
- Structural studies of fertilisation and zona pellucida module proteins – Luca Jovine's research group
- Tissue regeneration, focusing on fat cells (adipocytes) and obesity – Kirsty Spalding's Group
- Robert Månsson group
- Developmental and Regenerative Stem Cell Medicine – Lanner Lab
- Suicide and mental health lab – Vladimir Carli and Gergö Hadlaczky's group
- Medical, genetic and molecular decoding of neurodevelopmental disorders – Kristiina Tammimies' research group
- Working hours, Recovery and Safety in working life. – Anna Dahlgren's research group
- Research groups in hematology
- SOMIND: Somatic, Mental, and Neurodevelopmental Conditions – Agnieszka Butwicka's research group
- Research groups in drug abuse and addiction
- Research groups in biomaterials science
- Mikael Adner's research group
- Chemical and functional neuroanatomy of pain and stress systems – Tomas Hökfelt group
- Spider silk biology for biomedical applications – Anna Rising's research group
- Cancer Epidemiology and Causal Inference – Research group Maria Feychting
- Protein biochemistry for development of novel medical treatments – Janne Johansson's research group
- Healthcare financing and organization – Clas Rehnberg's group
- Space and environmental physiology – Rodrigo Fernandez-Gonzalo group
- Novel treatment approaches for neuroblastoma – Malin Wickström Näsman research group
- Cancer immune and gene therapy – Rolf Kiessling's Team
- Pancreas biology – Amanda Andersson Rolf's Group
- CAIR-LAB: Clinical AI Research Laboratory – group
- Atopic dermatitis – Research group Emma Johansson
- Reproductive endocrinology and metabolism – Angelica Lindén Hirschberg's research group
- Genetic basis for B and T cell recognition and function – Gunilla Karlsson Hedestam Group
- Cognitive Aging and Mental Health – Erika Jonsson Laukka Group
- Molecular and circuit neuropharmacology – Gilberto Fisone group
- The role of immunity in shaping adipose tissue development and metabolism – Jurga Laurencikiene group
- Chronic myeloid malignancies – Johanna Ungerstedt group
- Studies of tissue microbiology and immunology – Mattias Svensson group
- Sten Linnarsson group
- Balance, gait, exercise and physical activity in neurological diseases – Franzén Group
- Global Health Pharmacology and Therapeutics (GH-Pharma) – Eleni Aklillu's research group
- Real-world-data in Multiple Sclerosis – Anna Glaser's research group
- Suicidology and transcultural psychiatry – Marie Dahlin's research group
- Experimental and Clinical Neuroendocrinology – Maria Petersson's research group
- Cutting edge translational research of common and rare skin disease – Research group Jakob Wikström
- Alzheimer’s Disease Risk Factors & Sex-Specific Mechanisms – Silvia Maioli's research group
- Frontal lobes and cognitive failure – Wahlund's research group
- Clinical Pharmacology – Research group Rickard Malmström
- Molecular Vascular Medicine – Team Lars Maegdefessel
- Molecular endocrine pathology – Christofer Juhlin's Group
- Presynaptic mechanisms – Lennart Brodin group
- Malignant melanoma – Hildur Helgadottir's Group
- Precision strategies to eradicate heterogeneous tumor cells in pediatric cancer – Kasper Karlsson's Group
- Respiratory and systemic immune responses in human pulmonary viral infection and inflammation – Research group Anna Smed Sörensen
- Genetic toxicology – Kristian Dreij's group
- Identifying new molecular targets for the treatment of motor neuron diseases – Eva Hedlund's research
- Epigenetics - basic mechanisms and disease – Karl Ekwall's research group
- Forensic medicine – Henrik Druid's Group
- Ageing and Health – Karin Modig's research group
- Perception Neuroscience – Johan Lundström's research group
- Computational medicine and bioinformatics – Trung Nghia Vu's research group
- Toxicology – Bertrand Joseph´s Research group
- Etiology, prevention and treatment of eating disorders – Ata Ghaderi's research group
- Research groups in medical ethics and history of science
- Research groups in occupational and environmental health
- Research groups in surgery
- Research groups in biophysics
- Research Group Johan Ärnlöv
- Narrative in health and social care – Research group
- Cellular and molecular mechanisms underlying intervertebral disk formation and Diversity and plasticity of adipose tissue cell niche – Meng Xie's research team
- Neuroimmunology of cancer – Sébastien Talbot
- Pediatric Systems Immunology – Petter Brodin's research group
- Microvascular oxidative stress in the triad of cardiovascular, metabolic and renal disease – Mattias Carlström group
- Cardio-renal epidemiology – Juan-Jesus Carrero's research group
- Human Translational Genetics – Stefano Romeo Group
- Clinical Pancreatology – Miroslav Vujasinovic research group
- Hemostasis and Thrombosis – Mika Skeppholms research group
- Movement, Activity and Health – Eva Broström's research group
- Multidimensional health in old age – Amaia Calderón-Larrañaga group
- Molecular epidemiology of aging – Sara Hägg's research group
- Long noncoding RNA in the adipocyte – Alastair Kerr Research Group
- Leukemia niche – Hong Qian group
- Disease mechanisms in severe acute bacterial infections – Anna Norrby-Teglund group
- Gastrointestinal pediatric surgery – Tomas Wester's research group
- Developmental biology and regenerative medicine – Igor Adameyko's research group
- Experimental audiology – Barbara Canlon's research group
- Translational Immunology in Neuroinflammation – Olivia Thomas' research group
- Sickness absence, health and living conditions – Emilie Friberg's research group
- Health Systems and Policy – Cecilia Stålsby Lundborg's research group
- Endocrinology and autoimmune diseases – Research group Sophie Bensing
- Health economics of Alzheimer disease and other dementias – Linus Jönsson Group
- Epidemiology: Diet, contaminants and health – Agneta Åkesson's research group
- Neuronal circuits of anxiety – Janos Fuzik group
- Paediatric rheumatology – Research group Helena Erlandsson Harris
- Cardiovascular Epidemiology – Karin Leander research group
- Molecular cardiology – Research group Francesco Cosentino
- Björn Högberg group
- Regeneration mechanisms – Andras Simon's Group
- Assessing and managing chemical risks – Linda Schenk's research group
- Autoimmunity and adaptive immunity in rheumatic disease – Research group Vivianne Malmström
- Rheumatic systemic diseases – Team Marie Holmqvist
- Heart failure with reduced and preserved ejection fraction. Clinical and translational aspects. – Research group Lars Lund
- Christoph Ziegenhain Group
- Psychiatric epidemiology – Paul Lichtenstein's research group
- Structural basis of RNA biogenesis – Martin Hällberg's research group
- Hematopoietic Stem Cell Biology Group – Sten Eirik W. Jacobsen Lab
- Psychiatric Genetic Epidemiology – Sarah Bergen's research group
- Renal Medicine at Novum (Baxter Novum) – Bengt Lindholm Group
- Research groups in biochemistry
- Psychological treatment: internet and exposure – Brjánn Ljótsson's research group
- Research groups in immunology
- Research groups in ophthalmology
- Research groups in urology and nephrology
- Research groups in sociology and migration
- Integrative physiology – Juleen Zierath and Anna Krook
- Hormonal signalling and cancer – Cecilia Williams research group
- Cell Biology of Cancer – Staffan Strömblad's research group
- Chronic inflammation with focus on rheumatology and cancer – Research group Per-Johan Jakobsson
- Health services and social work research – Lena Von Koch's research group
- Nitrate-nitrite-NO pathway in health and disease – Eddie Weitzberg's research group
- Medical sensors and diagnostics – Team Onur Parlak
- Uncovering the molecular and physical principles governing early embryonic division and nuclear organization – Jan Ellenberg Group
- Nanoscale lipid flux architecture – Veijo Salo’s Research Group
- Residual conditions after polio and neuromuscular diseases – Eva Melin's research group
- Amyotrophic Lateral Sclerosis - ALS – Caroline Ingre's research group
- Saving the brain in critically ill neonates – Ulrika Ådén's research group
- Regulatory Transcriptomics – Kutter group
- NeuroimAging – Grégoria Kalpouzos group
- Section of Pharmacogenetics – MIS-lab
- Biological and clinical aspects of the myelodysplastic syndrome – Magnus Tobiasson group
- Human innate lymphoid cell lab – Jenny Mjösberg group
- Viruses and their interactions with the immune system – Sara Gredmark Russ group
- Protein degradation pathways – Helin Norberg's research group
- Translational Auditory Neuroscience – Christopher Cederroth's research group
- Neuroradiology: Neurodegeneration and Neuroinflammation – Tobias Granberg's research group
- Tumor Immunology and Immunotherapy Group – Dhifaf Sarhan
- Causal inference in epidemiologic research – Arvid Sjölander's research group
- The role of autophagy in Aβ metabolism and neurodegeneration in Alzheimer’s disease – Per Nilsson group
- Darreh-Shori's Lab/group
- PROCOME – Hanna Öfverström's and Marta Roczniewska's group
- Molecular brain imaging of neurodegenerative disorders – Andrea Varrone's research group
- Alcohol and Drug Epidemiology – Mats Ramstedt's research group
- Anxiety related disoders and genetics – Christian Rück's research group
- Psychotherapy Research – Tobias Lundgren's research group
- Human olfaction – Mats J. Olsson's research group
- Tissue stem cells and aging – Pekka Katajisto's Group
- SOSVASC – David Lindström's research group
- Yenan Bryceson group
- Epidemiology of sarcoidosis and systemic lupus erythematosus – Team Elizabeth Arkema
- Viral genomics and metagenomics – Tobias Allander Group
- Defining the metabolic signatures of tissue resident human ILCs – Chris Tibbitt team
- Human papillomavirus which causes cervical cancer – Research Group Karin Sundström
- Research groups in bioinformatics and systems biology
- Cognitive epidemiology – Bo Melin's research group
- Gilad Silberberg group
- Research groups in oto-rhino-laryngology and logopaedics
- Immunoregulation of environmental-induced airway inflammation in severe asthma and bronchiectasis – Apostolos Bossios's research group
- Multimorbidity and frailty in older adults – Davide Liborio Vetrano group
- Psychiatric and pharmaco-epidemiology – Zheng Chang's research group
- Charting normal and malignant hematopoiesis – Team Joakim Dahlin
- Molecular mechanisms governing oxygen homeostasis in development, evolution, and disease – Kragesteen's research group
- Precision Medicine – Infrastructure team
- Research groups in medical epigenetics and epigenomics
- Spinal circuits for whole-body motor coordination – Laurence Picton group
- Clinical HIV epidemiology and comorbidity – Christina Carlander Research group
- Head and neck pathology – Anders Näsman's Team
- Carin Håkansta's research group – Digital work environment and health
- Pediatric and respiratory epidemiology – Catarina Almqvist Malmros' research group
- Sexual and Reproductive Health – Marie Klingberg-Allvin research group
- Angiogenesis, Cancer, Metabolic disease, Cardiovascular disease, Eye disease – Yihai Cao Group
- Cardiovascular and psychosocial health in resilience and healthy brain aging – Chengxuan Qiu Group
- Translational Psychiatry – Catharina Lavebratt's research group
- Rare metabolic liver diseases, coagulation and hepatocellular carcinoma – Stål and Wahlin research group
- Blood Engineering – Vanessa Lundin group
- Development, regulation and function of human innate lymphoid cells throughout life – Jakob Michaëlsson group
- Clinical Genetics – Richard Rosenquist Brandell's research group
- Oxysterols – Ingemar Björkhem and Ulf Diczfalusy research
- Molecular exercise physiology – Carl Johan Sundberg's research group
- Large-scale Network Connectivity in the Human Brain – Peter Fransson's research group
- Environmental physiology
- The Systems Virology Lab – Ujjwal Neogi
- Cancer Cell Invasion – Marco Gerling's research group
- Molecular brain imaging, neuropsychopharmacology, depression and anxiety disorders – Johan Lundberg's research group
- Organization and operation of postural neuronal networks in health and disease – Tatiana Deliagina group
- Paediatrics – Anna Lindholm Olinder's research group
- Petzold group
- Molecular and epidemiological studies of ascending aortic aneurysms – Research group Hanna Björck
- Prevention, Intervention and Mechanisms in Public Health (PRIME Health) – Cecilia Magnusson's research group
- The interplay between circadian 3D genome organization and metabolism in complex diseases – Anita Göndör's Group
- Reconstructive Plastic Surgery and Global Surgery – Jenny Löfgren's research group
- Real-world effectiveness of psychopharmacological treatment – Jari Tiihonen's Research Group
- Center for Heart Failure and Arrhythmia – Gianluigi Savarese
- Sports Medicine – Anders Stålman's research group
- Women's Mental Health Epidemiology – Donghao Lu Lab
- The SMC complexes, chromosome dynamics and stability – Camilla Björkegren's Group
- Extracellular vesicles in immunity – Research group Susanne Gabrielsson
- Precision Cancer Medicine in Lung Cancer - Preclinical, Translational & Clinical Research – Simon Ekman's research group
- RNA-guided DNA repair and cancer – Marianne Farnebo's research group
- Laboratory of translational fertility preservation – Kenny Rodriguez-Wallberg's group
- Research groups in cancer and oncology
- Common Mental Disorders and Behavioral Medicine in Primary Care – Erik Hedman's research group
- Neuronal membrane trafficking – Oleg Shupliakov group
- Research groups in paediatrics
- Biostatistics With Focus on Statistical Methods in Cancer Epidemiology and Screening – Keith Humphreys' research group
- Research groups in nanotechnology
- Gastroenterology – clinical epidemiology and clinical trials – Research group Ola Olén
- Precision engineering of T cells in vivo for therapies of cancer – William Nyberg team
- Protein Misfolding and Prevention by Molecular Chaperones in Cancer and Neurodegenerative Disease – Gefei Chen's research group
- Social Perception – Arvid Guterstam's research group
- Neuroepidemiology – Kyla McKay and Katarina Fink's research group
- Experimental growth research – Lars Sävendahl's research group
- Neuronal circuits of the Basal Ganglia – Maya Ketzef group
- Psychological Interventions - innovation, improvement and implementation – Viktor Kaldo's research group
- Susanna Ranta team
- Neurovascular diseases – Christina Sjöstrand research group
- Lifestyle, Prevention and Health – Ylva Trolle Lagerros research group
- Elias Arnér research group
- Gynecologic Cancer – Henrik Falconer's research group
- B cell biology, from immunodeficiency to cancer – Pan Hammarström Lab
- Developmental Cognitive Neuroscience – Torkel Klingberg group
- Rare Diseases – Ann Nordgren och Anna Lindstrand's research group
- Research on steatotic liver disease – Hagström Group
- Stem Cell Size – Jette Lengefeld Team
- Natural Killer Cell Biology and Cell Therapy – Kalle Malmberg group
- Mast Cell Biology – Research group Gunnar Nilsson
- Traumatic Brain Injuries and Neuromonitoring – Eric Thelin's research group
- Medical Statistics team
- Augmented Neurosurgery – Erik Edström and Adrian Elmi Terander's research group
- Molecular neurophysiology – Karima Chergui's research group
- Injuries Social Aetiology and Consequences (ISAC) – Lucie Laflamme's research group
- Unconventional T cells in human pathology – Andrea Ponzetta team
- Inflammation and immune regulation during infection – Team Christopher Sundling
- Orthopaedics – Anders Enocson's research group
- Endothelial dysfunction in atherosclerotic cardiovascular disease – Research Group John Pernow
- Clinical epidemiology of multiple sclerosis and inflammatory joint diseases – Team Thomas Frisell
- Cancer stem cells and clonal structure in acute lymphoblastic leukemia – Martin Enge's Group
- Mitochondrial metabolism in health and disease – Anna Wredenberg group
- Biomolecular Medicine and Advanced therapies- focus on delivery of RNA therapeutics – Research group Samir EL Andaloussi
- Antimicrobial resistance – Christian Giske research group
- Tissue immunology – Research group Helen Kaipe
- Health Economics and Economic Evaluation – Niklas Zethraeus' group
- Mental Health Neural Dynamics Lab – Kristoffer Månsson's research group
- Stem Cell Biology – Jonas Muhr's Group
- VIVAC research group: Vaccines and Immunotherapies against Viruses And Cancer – Matti Sällberg
- The passage of the mRNP particle through the nuclear pore – Bertil Daneholt's research
- Therapeutic Immunology and Transfusion Medicine – Michael Uhlin’s research group
- Musculoskeletal conditions and sports medicine research – Iben Axén's research group
- Research groups in cancer and oncology
- Nutritional neuroscience – Janina Seubert's Research Group
- Tobias Karlsson's group
- Research groups in pharmacology and toxicology
- Precision Psychiatry – Lu Yi's research group
- Research groups in medicinal chemistry
- Agneta Richter-Dahlfors group
- Cancer epidemiology with focus on breast cancer and applied biostatistics – Anna Johansson's research group
- Autism, ADHD and other neurodevelopmental conditions – Sven Bölte's research group
- Twinstudies on health conditions and sickness absence – Pia Svedberg's research group
- Psychiatric comorbidity and intergenerational transmission of mental health problems – Erik Pettersson's research group
- Genetic and pharmacological epidemiology – Zeberg laboratory
- REACH – digital treatment and prevention – David Moulaee Conradsson's research group
- Mucosal immunology lab – Charlotte Thålin research group
- Stroke – Annika Lundströms research group
- Loh
- Gonçalo Castelo-Branco Group
- Pediatric Neuroimmunology and Epilepsy – Ronny Wickström's research group
- Explores various cell signaling phenomena and their impact on critical biological and medical processes using advanced light microscopy – Per Uhlén Group
- Neural stem cells – Anna Falk group
- Diabetes and Associated Complications – Sergiu-Bogdan Catrina's research group
- Myelodysplastic syndromes – Eva Hellström Lindberg group
- Immune responses to human viral infections and cancer – Hans-Gustaf Ljunggren group
- Health services research in neurological conditions – Charlotte Ytterberg's research group
- Neurosurgery – Mikael A. Svensson's research group
- Westman neuroimaging group
- Stemcells and Inflammation – Lou Brundin's research group
- Pharmacological nitric oxide research – Jon Lundberg's research group
- Epidemiology of Psychiatric Conditions, Substance use and Social Environment (EPiCSS) – Emilie Agardh's & Renee Gardner's Research Group
- Autoimmune neurology – Jakob Theorell team
- Reducing antibiotic resistance – Research group Pontus Nauclér
- Orthopaedics – Karl Eriksson's research group
- Endocrine Surgery – Robert Bränström's research group
- Paediatrics – Inger Kull's research team
- Cancer proteogenomics - from methods to clinical applications – Janne Lehtiö's Group
- Primary Immunodeficiency, Innate Immunity and Antimicrobial peptides – Peter Bergman Research Group/The AMP-group
- Clinical Virology and Immunology – Annika Karlsson's research group
- Jussi Taipale Group
- Global Child Health and the Sustainable Development Goals – Tobias Alfvén's research group
- Topoisomerases, chromatin biology and cancer – Laura Baranello's group
- Stem cells and neural development – Johan Ericson's Group
- Vascular morphogenesis and function in health and disease – Lars Jakobsson group
- Epidemiology and Public Health Intervention Research (EPHIR) – Jette Möller's research group
- Epithelial stem cells in development and disease – Maria Genander's group
- Research Group Gustaf Edgren
- Drug discovery, pancreatic beta-cell regeneration – Olov Andersson's Group
- Unit of Biostatistics – Matteo Bottai
- Research groups in cardiology and cardiovascular diseases
- Sleep, cognition and health – John Axelsson's research group
- Research groups in infectious medicine
- Research groups in physiology and anatomy
- Cardiometabolic epidemiology and aging – Ida Karlsson's research group
- How the overview of research fields is made
- Precision Cancer Control Group – Martin Eklund's research group
- Liver and monocyte remodelling in non-alcoholic fatty liver disease and cardiovascular disease – Rongrong Fan's research group
- Early childhood development and neurodevelopmental conditions – Terje Falck-Ytter's research group
- Mental health and social integration (Mente) – Ellenor Mittendorfer-Rutz's research group
- Mechanisms of protein aggregation and inhibition – Axel Abelein group
- Accelerating drug discovery using molecular modeling and machine learning – Andreas Luttens' group
- Pediatric Healthcare Science – Cecilia Bartholdson research group
- Digital Strategies for AF Detection and Outcome Prevention – Emma Svennberg Team
- Speech-Language Pathology – Liza Bergström's research group
- Arrhythmia – Johan Engdahl's research group
- Environment, nutrition and health – Anna Bergström's research group
- Genetic and epigenetic factors in asthma and allergy – Cilla Söderhäll's research group
- Cardiovascular epidemiology – Team Bruna Gigante
- Neurobiology of Stress and Treatment Response – Juan Pablo Lopez group
- Vascular Surgery – Ulf Hedin's research group
- Annika Bergquist group
- Acute myeloid leukemia – Sören Lehmann group
- Human tissue-resident NK cells – Niklas Björkström group
- Neuropsychoimmunology – Sophie Erhardt's research group
- Diabetes epidemiology – Sofia Carlsson's research group
- Stroke - Acute Intervention and Secondary Prevention – Niaz Ahmed's research group
- Molecular muscle physiology and pathophysiology – Lanner Lab
- Community nutrition and physical activity (CoNPA) – Liselotte Schäfer Elinder's research group
- Inflammatory bowel disease (IBD) – Research group Eduardo Villablanca
- CRISPR-based drug target discovery in cancer and autoimmunity – Research group Fredrik Wermeling
- Emergency care – Kristian Ängeby's research group
- Clinical Cancer Epidemiology – Research group Karin Ekström Smedby
- Cancer prevention and screening – Johannes Blom's research group
- Somatosensation – Patrik Ernfors group
- DNA replication & Cancer Genetics – Lemmens Group
- Laboratory testing for alcohol and drugs of abuse – Anders Helander research
- Anaesthesia and Intensive care – Rebecka Rubenson Wahlin/Anna Schandl's research group
- Jonas Fuxe Group
- SCF ubiquitin ligases, cell cycle, transcription and cancer development – Olle Sangfelt's Group
- Toxicological Mechanisms – Emma Wincent's research group
- Stem Cells in Tissue Homeostasis and Regenerative Medicine – Jonas Frisén's Group
- Urology – Olof Akre's research group
- The New World of Work – Theo Bodin's research group
- Midbrain dopaminergic neuron development
- Research group Michael Fored
- Neurobiology of pain & Therapeutics – Saida Hadjab Group
- Risk assessment – Mattias Öberg's research group
- Research groups in cell and molecular biology
- Developmental Psychology: Digital Media and ADHD – Lisa Thorell's research group
- Research groups in education and pedagogy
- Research groups in biostatistics and probability theory
- ESSI – Emotion regulation, Self-injury, Suicide, and Intervention – Johan Bjureberg's research group
- Medical ethics – Gert Helgesson's group
- Treatment of substance use disorders – Johan Franck's Research Group
- Sex hormones and sex differences in diseases of the brain – Ivan Nalvarte's research group
- Cilia in the brain – and their connections to human brain disorders – Peter Swoboda's research group
- Evidence-based methods in autism and ADHD – Tatja Hirvikoski's research group
- Long-Term Outcomes after Perioperative and Intensive Care – Max Bell research group
- Translational and clinical research in acute myeloid leukemia – Team Martin Jädersten
- CEREBRA – Susanne Palmcrantz's research group
- Ute Römling group
- Perioperative quality – Jan Jakobssons research group
- Translational Pharmacology – Kent Jardemark's team
- Global and Sexual Health (GloSH) – Anna Mia Ekström's research group
- Medical Inflammation Research – Rikard Holmdahl group
- Neuro-infections & Neuroinflammation – Federico Iovino Group
- Clinical Chemistry and Blood Coagulation – Jovan Antovic's research group
- Translational research on human microbial infections and its consequences – Anders Sönnerborg's group
- Human tissue-resident NK cells in homeostasis and disease – Nicole Marquardt team
- Etiology and pathogenesis of type 1 diabetes – Malin Flodström-Tullberg group
- Oxygen sensing, cancer and intratumor heterogeneity – Schlisio Lab
- Genetic Epidemiology of Neuroinflammatory Disorders – Ingrid Kockum's research group
- Retina – Anders Kvanta's research group
- Glaucoma – Pete Williams' research group
- Leadership in healthcare and academia – Mia von Knorring's group
- Luminal gastroenterology – Research group Charlotte Hedin
- Identifying molecular signals in the genital mucosa that determine susceptibility to sexually transmitted infections – Research group Kristina Broliden
- Coronary heart disease – Research group Per Svensson
- Educational development – Matti Nikkola's group
- Post-transcriptional regulation in mitochondria – Joanna Rorbach group
- Cellular diversity is generated during development, neuronal identity – Johan Holmberg's Group
- Lipid handling in health and cardiometabolic disease – Research group Carolina Hagberg
- Renal glomerulus biology and diseases – Jaakko Patrakka group
- Research group Caroline Palm Apergi
- Precision pathology and tumor heterogeneity – Johan Hartman's Group
- Lifelong Learning in Health Care Contexts – Terese Stenfors' group
- Translational Arthritis Research – Team Bence Réthi
- Stem cells, Developmental Biology, Heart Disease, Heart Development, Genetics, Biotechnology – Kenneth R. Chien's group
- Urological cancer – Lars Egevad's Group
- The ubiquitin/proteasome system in neurodegenerative diseases and cancer – Nico Dantuma's Group
- Experimental alcohol- and drug dependence research – Vladana Vukojević research group
- Chronic inflammatory disease epidemiology – Research group Johan Askling
- Statistical and bioinformatics analyses of high-throughput molecular data – Yudi Pawitan's research group
- Urban environment and children´s and youth health – Olena Gruzieva's research group
- Research groups in laboratory medicine
- Research groups in medical biotechnology
- Research groups in physiotherapy
- Breast cancer epidemiology – Kamila Czene's research group
- Research groups in human computer interaction
- Research group Liv Eidsmo
- Preventive Medicine – Susanna Larsson's research group
- Transition to adulthood for individuals with neurodevelopmental conditions – Ulf Jonsson's team
- Function and Health in Respiratory and Cardiovascular Conditions – Malin Nygren-Bonnier's research group
- Laboratory of lymphocyte biology – Team Taras Kreslavskiy
- Global Reproductive Outreach & Wellness (GROW) – Michael Wells
- Mats Marshall Heyman research group
- SLE/APS/Vasculitis group – Research group Aleksandra Antovic
- Itziar Martinez Gonzalez group
- CoNFIND Cognition and Fatigue – Marika Möllers research group
- Tissue immunosurveillance by cytotoxic lymphocytes – Takuya Sekine team
- Physical activity and Sports medicine with focus on prevention – Hagströmer research group
- Receptor biology and signaling – Schulte Lab
- Neurobiology of Obesity – Alessandro Furlan group
- Upper GI Surgery – Jesper Lagergren's research group
- Sepsis and COVID-19 – Kristoffer Strålin Group
- Harnessing Neutrophils for Precision Cell Therapy against solid tumors – Roland Fiskesund Team
- Human T cell immunity to evolving viruses and cancers – Marcus Buggert group
- Neuroradiology - MRI physics – Stefan Skare's research group
- Wilhelm lab
- Inflammatory responses in cancer and autoimmunity – Mikael Karlsson's Group
- Cultural Medicine – Solvig Ekblad's unit
- Working life, ergonomics, psychosocial factors, and health – Research group Daniel Falkstedt
- Aging and health – Welmer's research group
- NK Cell Recognition – Klas Kärre Group
- Computational Breast Imaging – Fredrik Strand's Group
- Drug treatment – Erik Eliasson research group
- Neural differentiation as a strategy for neuroblastoma treatment – Marie Arsenian Henriksson Group
- Cancer immunotherapy – Mattias Carlsten group
- Cell-based immune therapy for cancer – Andreas Lundqvist's Group
- Basic molecular structure of human skin – Lars Norlén's research
- Reproductive, Perinatal and Pediatric Epidemiology – Research group Olof Stephansson
- Research group Erik Melén
- Research group Joel Nordin
- MINT – Klas Karlgren's team
- Translational breast cancer research – Theodoros Foukakis' Group
- Genes function and molecular bases of diseases – Research group Magdalena Paolino
- Homeostasis and tissue repair mechanisms in the mammalian system, pericytes, stem cells – Christian Göritz Group
- The Perinatal Epidemiology Lifecourse Lab – Team Neda Razaz
- Experimental Cancer Medicine (ECM)
- Research group Therese Djärv
- Molecular and cellular neuroendocrinology – Tibor Harkany group
- Neuropsychiatric disorders – Håkan Karlsson group
- Research groups in dermatology and venereal diseases
- Evidence-based practice: prevention, intervention and implementation – Pia Enebrink's research group
- Research groups in radiology and medical imaging
- Research groups in psychiatry
- Maria Eriksdotter research group
- Research groups in precision medicine
- Breast cancer surgery – Jana de Boniface's research group
- ICare – Ann Langius-Eklöf's research group
- Immunology and Chronic Disease – Johan Frostegård's research group
- Health in Everyday Life (HELD) – Susanne Guidetti's research group
- Center for Resuscitation Science – Jacob Hollenberg's research group
- Applied Developmental Neurobiology – Carl Sellgren research group
- Reproductive Health/Reproductive Medicine – Kristina Gemzell Danielsson's research group
- Malin Holzmann's research group
- Radiation therapy/ Radiation Oncology – Pehr Lind Group
- Fossati
- APP processing and Abeta localization at super-resolution and glycan biomarkers for Alzheimer disease – Sophia Schedin Weiss's research group
- ALGOSH: Algorithmic Management at Work
- Development of autonomic control – Herlenius Research
- Maintenance and expression of mtDNA in disease and ageing – Nils-Göran Larsson Group
- Neuropharmacology - Movement Disorders – Per Svenningsson's research group
- Somatosensation & Gargalesis – Konstantina Kilteni group
- Signal Transduction – Ismael Valladolid Acebes' research group
- Microbiome and Infections – Piotr Nowak Research Group
- Immunological tolerance and transfusion immunology – Petter Höglund group
- Immunopathogenesis and new therapeutic strategies in tuberculosis – Susanna Brighenti group
- Pain and Brain Imaging – Karin Jensen's research group
- Susanne Nylén group
- Hand surgery – Maria Wilckes Research group
- Environmental impact on pulmonary host defence and chronic airflow obstruction – Anders Lindén's research group
- Rehabilitation, collaboration and aging – Elisabeth Rydwik's research group
- Tuberculosis Aerobiology – Antonio Rothfuchs group
- Oscar Fernandez-Capetillo group
- Diversity within the T cell response, T cell memory – Research group Carmen Gerlach
- Clinical and translational melanoma research – Hanna Eriksson's Group
- Meiotic chromosome segregation – Christer Höög's research group
- B cells and autoantibodies in rheumatic disease – Team Caroline Grönwall
- Precision medicine in lymphoid malignancies including CLL; from new targets to real-world assessments – Anders Österborg's Group
- Research group for Cancer evolution
- Jiri Bartek group
- Translational Cardiology (@TransCardio) – Research group Magnus Bäck
- Gene regulation, genome-wide experimental and computational techniques – Rickard Sandberg's Group
- The Helleday Laboratory focuses on harnessing defects in the DNA damage response and metabolism to develop novel therapies
- Small RNAs in cancer development – Weng-Onn Lui's Team
- Geriatric pharmacoepidemiology – Kristina Johnell's research group
- Molecular neurodevelopment and neuro-oncology – Ola Hermanson group
- Research groups in anesthesiology and intensive care
- Gastrointestinal epidemiology – Jonas Ludvigsson's research group
- Health inequalities and minority stress – Richard Bränström's research group
- Research groups in microbiology
- Research groups in orthopaedics
- Hereditary hematological malignancies – Bianca Tesi team
- Lars Holmgren's Group
- Substance use prevention – Johanna Gripenberg's research group
- Perioperative care – Ulrica Nilsson's research group
- Mechanisms behind HIV-1 latency and rebound – Peter Svensson's group
- ENGAGE- Enacting health and change through everyday activity – Patomella group
- Diagnosis and treatment of children with asthma and allergy: from molecular diagnosis to intervention – Jon Konradsen's research group
- Translational research in childhood cancer and histiocytic diseases – Nikolas Herold Research group
- Coronary artery disease – research at Danderyd hospital
- Mechanisms of malignant hematopoiesis – Pedro Moura team
- Regulation of B cell activation – Pia Dosenovic Group
- Complex interventions – Team Carina King
Medical Inflammation Research – Rikard Holmdahl group
Our research group, led by Rikard Holmdahl, aims to understand the basic pathogenic mechanisms behind autoimmune diseases and cancer. We try to identify and functionally analyse the genes that control autoimmune diseases, primarily by using animal models in which it is possible to identify genetic causes of complex diseases. With this knowledge we develop new diagnostics and vaccines for the treatment of autoimmune diseases like rheumatoid arthritis, and aim to improve immunotherapy in cancer.
Open positions
We are currently looking for two post docs. Apply no later than 29 January.
Postdoctoral Researcher in Regulation of autoreactive B cells by Ncf1 polymorphism
 Photo: Stefan Zimmerman
Photo: Stefan ZimmermanBiomedicum
Our division is located within Biomedicum, a research building that houses a large part of the experimental research conducted at Karolinska Institutet. The building opened its doors in 2018 and promotes collaboration and research across scientific boundaries.
Publications
Selected publications
- Article: ADVANCED SCIENCE. 2024;11(23):e2401513Romero-Castillo L; Li T; Do N-N; Sareila O; Xu B; Hennings V; Xu Z; Svensson C; Oliveira-Coelho A; Sener Z; Urbonaviciute V; Ekwall O; Burkhardt H; Holmdahl R
- Review: ANNALS OF THE RHEUMATIC DISEASES. 2024;83(5):550-555He Y; Aoun M; Xu Z; Holmdahl R
- Article: JOURNAL OF EXPERIMENTAL MEDICINE. 2023;220(11):e20230101Aoun M; Coelho A; Krämer A; Saxena A; Sabatier P; Beusch CM; Lönnblom E; Geng M; Do N-N; Xu Z; Zhang J; He Y; Romero Castillo L; Abolhassani H; Xu B; Viljanen J; Rorbach J; Fernandez Lahore G; Gjertsson I; Kastbom A; Sjöwall C; Kihlberg J; Zubarev RA; Burkhardt H; Holmdahl R
- Article: NATURE COMMUNICATIONS. 2023;14(1):5949Xu Z; Xu B; Lundstrom SL; Moreno-Giro A; Zhao D; Martin M; Lonnblom E; Li Q; Kramer A; Ge C; Cheng L; Liang B; Tong D; Stawikowska R; Blom AM; Fields GB; Zubarev RA; Holmdahl R
- Article: ARTHRITIS & RHEUMATOLOGY. 2023;75(7):1110-1119Lonnblom E; Leu Agelii M; Sareila O; Cheng L; Xu B; Viljanen J; Hafstrom I; Andersson MLE; Bergstrom G; Ekwall A-KH; Rudin A; Kastbom A; Sjowall C; Jacobsson LTH; Kihlberg J; Gjertsson I; Holmdahl R
- Article: PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 2023;120(25):e2218668120Urbonaviciute V; Romero-Castillo L; Xu B; Luo H; Schneider N; Weisse S; Do N-N; Oliveira-Coelho A; Fernandez Lahore G; Li T; Sabatier P; Beusch CM; Viljanen J; Zubarev RA; Kihlberg J; Bäcklund J; Burkhardt H; Holmdahl R
- Article: ANNALS OF THE RHEUMATIC DISEASES. 2023;82(6):799-808Li T; Ge C; Kramer A; Sareila O; Agelii ML; Johansson L; Forslind K; Lonnblom E; Yang M; Xu B; Li Q; Cheng L; Bergstrom G; Fernandez G; Kastbom A; Rantapaa-Dahlqvist S; Gjertsson I; Holmdahl R
- Article: NATURE COMMUNICATIONS. 2023;14(1):691A subset of antibodies targeting citrullinated proteins confers protection from rheumatoid arthritisHe Y; Ge C; Moreno-Giro A; Xu B; Beusch CM; Sandor K; Su J; Cheng L; Lonnblom E; Lundqvist C; Slot LM; Tong D; Urbonaviciute V; Liang B; Li T; Lahore GF; Aoun M; Malmstrom V; Rispens T; Ernfors P; Svensson CI; Scherer HU; Toes REM; Gjertsson I; Ekwall O; Zubarev RA; Holmdahl R
- Article: ARTHRITIS & RHEUMATOLOGY. 2022;74(6):961-971Ge C; Tong D; Loennblom E; Liang B; Cai W; Fahlquist-Hagert C; Li T; Kastbom A; Gjertsson I; Dobritzsch D; Holmdahl R
- Article: ANNALS OF THE RHEUMATIC DISEASES. 2022;81(4):480-489Ge C; Weisse S; Xu B; Dobritzsch D; Viljanen J; Kihlberg J; Do N-N; Schneider N; Lanig H; Holmdahl R; Burkhardt H
Staff and contact
Group leader
- Rikard HolmdahlAffiliated to Research
All members of the group
- Changrong GeAffiliated to Research
- Shamasree GhoshAffiliated to Research
- Angel Yao MattissonProject Coordinator
Current research
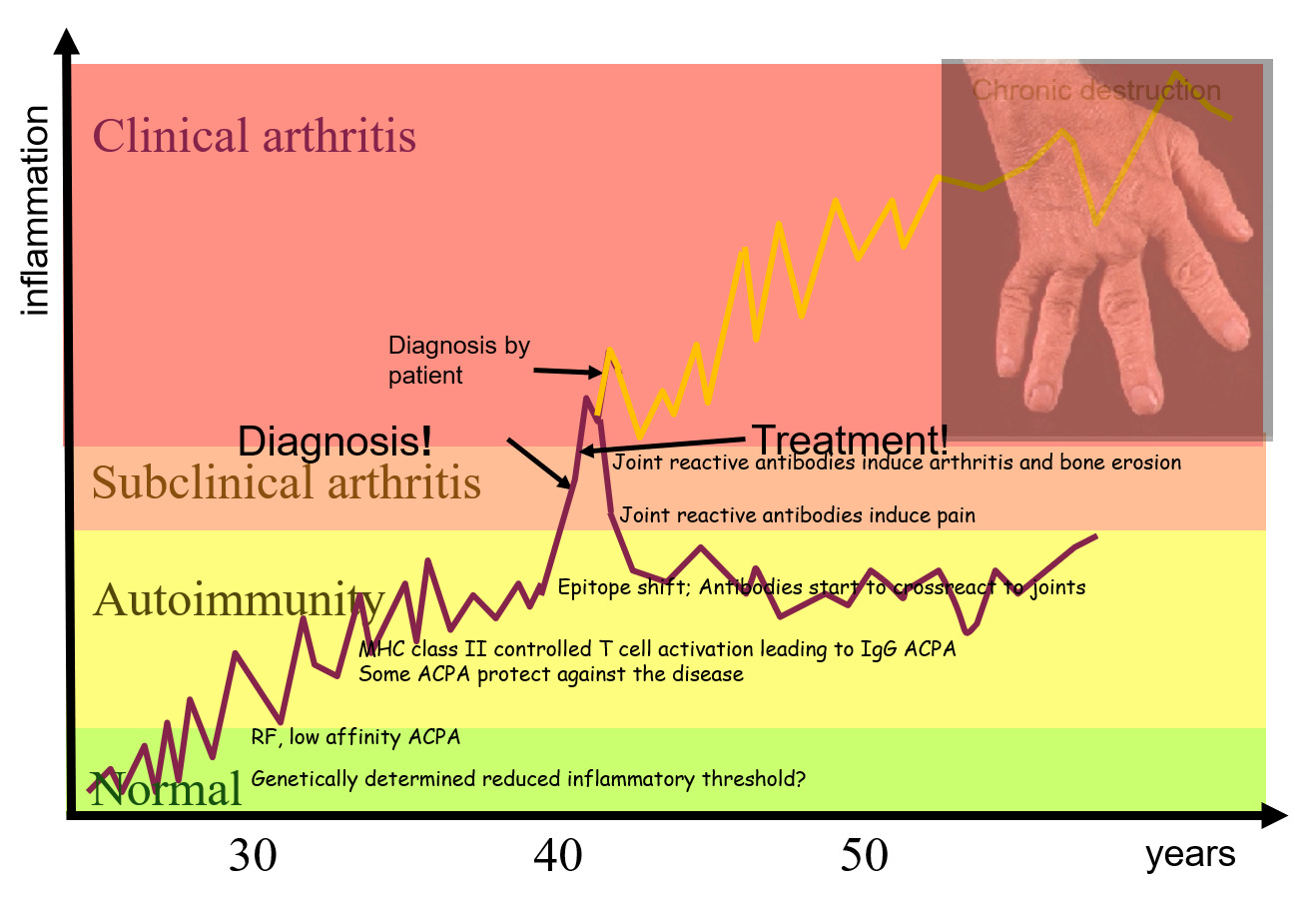
Open Diagnostics project: JointID clinical treatment research – a new unique diagnostic test for Rheumatoid Arthritis configuration options
Find out below what the research within the division of Medical Inflammation Research (MIR) is aimed at. Also listed are examples of research projects and an overview of our collaborations and recent publications.
The aims of our research are:
- To identify and functionally analyse the genes that control animal models for chronic inflammatory diseases, mainly using models for rheumatoid arthritis.
- To use the animal models not only for understanding the basic mechanism of autoimmune disease and for developing new diagnostic, preventive and therapeutic strategies.
- To understand the role of MHC class II genes in explaining the immune specificity of autoimmune disease.
We have different research projects within our division. Below, some examples of such projects.
Diagnostics project: JointID clinical treatment research – a new unique diagnostic test for Rheumatoid Arthritis
Inflammation in cartilaginous joints occurs in many common diseases such as rheumatoid arthritis (RA), osteoarthritis (OA) and psoriasis arthritis (PsA). It is known that antibodies to modified proteins (anticitrullinated protein antibodies and rheumatoid factors) are useful for predicting and classifying RA. It has however been difficult to identify specific and useful biomarkers derived from the joint inflammation itself.
We have focused on the immune response to joint cartilage proteins, in particular type II collagen (CII). This is directed to exposed triple helical structures, as well as to structures modified e.g. by citrullination. The response is connected with the onset of the RA and provides very sensitive biomarkers for joint inflammation.
We have three main goals:
- To develop a multiplex antibody diagnostic kit with post-translationally modified (citrullinated, carbamoylated, nitrosylated, oxidized and glycosylated) and conformational protein epitopes. These epitopes are selected to be of importance for development of arthritis in animal models. The purpose is to allow diagnosis of RA before disease onset and to help in determination of the type of therapy. The assay will also be used to select RA patients suitable for vaccination.
- To develop a diagnostic kit to identify T cells specific for glycosylated CII. It will be used to predict and monitor vaccination effects of pre-RA and RA patients.
To extend the antibody kit with additional joint-related epitopes useful for diagnosing additional joint inflammatory diseases, including common diseases like OA and PsA
Coordinator

Main PI
Prof. Rikard Holmdahl.
Cooperation
Prof. Inger Gjertsson, Göteborg University; Prof. Jan Kihlberg, Uppsala University
Supported by
- Swedish Foundation for Strategic Research (SSF) 2015 – 2020 (Dnr: RB13-0156, 35,000,000 SEK)
Vaccine project: Developing vaccines to cure autoimmune diseases
The next step in the treatment of autoimmune disease will be to prevent and cure, rather than only treating the symptoms. We focus on vaccine development to both prevent and treat rheumatoid arthritis (RA) but subsequently also other autoimmune diseases.
Vaccines have not yet been developed to any autoimmune disease, but we have several unique findings that will be tested clinically in RA. Our patent protected approach is to induce regulatory T cells by administration of a protein complex with the product of a strongly associated RA gene together with a protein fragment from collagen type II in joint cartilage that is a recognized by T cells in RA.
We have established new unique mouse strains with the human genes of interest, allowing development and validation of new vaccines not only for RA but for other autoimmune diseases as well. We have also developed new technology that allows modification of peptide structure; glycosylation and protein carrier, to develop improved personalized variants vaccines.
Coordinator
Main PI
Prof. Rikard Holmdahl
Cooperation
Prof. Roman Zubarev, Division of Chemistry I and chemical proteomics platform, Karolinska Institutet; Prof. Jan Kihlberg, Uppsala University; Dr. Kajsa Wing, Division of Medical inflammation Research, Karolinska Institutet
Supported by

- Swedish Science Council (VR), 2018-2022, (Dnr. 2017-06104, 19,000,000 SEK)
- Familjen Erling-Perssons Stiftelse, 2018-2020 (2017-10-09, 9,000,000 SEK)
- Novo Nordisk Foundation, 2025-2027 (0090035, 5,953,250 DKK)
Ncf1 project: Changing the view on autoimmune disease based on positional cloning of the Ncf1 gene
We have identified several major causative polymorphisms in mouse and rat models of autoimmune disease and are now investigating their functional role. One of these projects concerns Ncf1, a component of the NOX2 complex, which induces reactive oxygen species (ROS).
We found that Ncf1 alleles causing low production of reactive oxygen species (ROS) is a major cause of autoimmune disease as well as being a pronounced risk behavior, explaining the natural selection. In addition ROS is likely to regulate the immune response to cancer cells.
The Ncf1 locus is highly polymorphic and has in fact not been sequenced in humans, due to copy number variation, and is therefore not included in genome wide association studies. However, we have found that copy number variation and amino acid polymorphism is strongly associated with both rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).
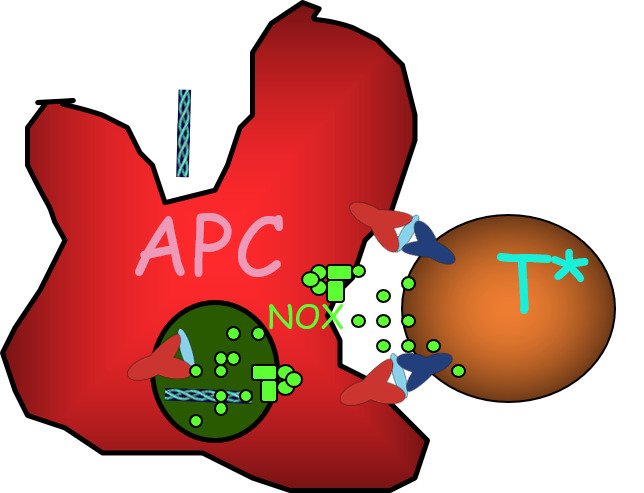
Based on our original finding and many years of combined efforts in both experimental animals and humans we have now established a unique platform and strategy to reveal a critical part of the pathogenesis of autoimmune disease. We have established CRISPR mutated and conditionally controlled Ncf1 mouse strains.
With models for RA and SLE we have also confirmed that the pathways are conserved between mice and humans. We have preliminary evidence of direct links to major pathways, such as interferon signaling and T and B cell receptor signaling. We intent to identify the immune regulatory cells expressing Ncf1 that play a critical role in the downstream pathway of autoimmune disease such as RA and SLE, but also in cancer. Also to determine the signaling oxidative and antioxidant pathways in the regulatory Ncf1 expressing cells as well as in cells exposed to secreted ROS.
This project will provide new insights into key pathogenic mechanisms based on redox regulation, leading to autoimmune and malignant diseases.
Coordinator
Main PI
Prof. Rikard Holmdahl
Cooperation
Prof. Elias Arnér, Division of Biochemistry, Karolinska Institute; Prof. Roman Zubarev, Division of Chemistry I and chemical proteomics platform, Karolinska Institute
Supported by

- Knut och Alice Wallenbergs Stiftelse, 2020 – 2024 (Dnr KAW 2019.0059, 36,500,000 SEK)
Details about the project funding: "Genfynd kan bana väg för vaccin mot autoimmuna sjukdomar" - Swedish Science Research Council (VR), 2020-2024 (Dnr. 2019-01209, 9,000,000 SEK)
Psoriasis vulgaris project: New therapy and diagnostics of psoriasis vulgaris and psoriatic arthritis based on new animal models
Psoriasis vulgaris (PsV) is an immune mediated disease of the skin, which is associated in one third of the patients with the development of psoriatic arthritis (PsA). PsV is a complex chronic disease driven by adaptive and innate lymphocytes involving the IL-17/22/23 signaling pathway. It is associated with the PSORS1 locus within the major histocompatibility complex (MHC) region, containing non-classical MHC genes regulating IL-17 producing innate lymphocytes. Besides the strong influence of the MHC region, a locus with the inducible nitric oxide synthase (NOS2) gene is associated with PsV/PsA susceptibility and the NOS2 locus regulate the exposure of various reactive oxygen species (ROS). Thus, genetic evidence argues for that non-classical MHC genes involved in the innate immune responses are regulated by RNS/ROS mediated functions.
The environmental causes of the PsV/ PsA in humans is not known but some sort of environmental challenges triggering the immune system in pathogenic way is likely. And it is likely that an immune adjuvant type of exposure, mediated by infections, pollution or smoking, will trigger or promote disease. Our project is based on the unique discovery that mannan exposure could induce the development of a disease in mice mimicking PsV and PsA. In this project, we will use the MIP model to investigate PsV and PsA with the aim to develop new type of diagnostics and treatments.
The mechanisms by which mannan triggers disease is not known but is likely involving activation of inflammatory cells through CLRs. These receptors could be both inflammatory and anti-inflammatory, partially dependent on their ability to activate the NOX2 complex that could induce reactive oxygen species (ROS), protecting disease development in the MIP model. Based on this, we will use mouse strains with and without the Ncf1 mutation to determine whether the observed therapeutic effect is dependent of the proposed mechanisms of action through the NOX2 complex. We will also use the model to facilitate the development of better diagnostics for PsV and PsA, with developed a multiplex peptide-based antibody test to investigate the ongoing autoantibody responses in in autoimmune diseases.
Coordinator
Zhongwei Xu
Postdoctoral researcherMain PI
Prof. Rikard Holmdahl
Supported by
- Leo Foundation 2023- 2025 (LF-OC-22-001023, DKK 3,622,500)
- Psoriasisförbundet 2021-2022 (2021-2022, 200,000 SEK)
See all our publications on PubMed and Google Scholar
Collaborations
- Partner in network for Cancer Redox - targeting redox pathways for improved cancer therapy, Coord. Prof. Elias Arnér, Biochemistry, Karolinska Institute, Supported by Knut och Alice Wallenbergs Stiftelse, 2016 – 2021 (Dnr. 2015.0063)
- Partner in network for A new unique diagnostics test for Rheumatoid Arthritis, Coord Prof. Inger Gjertsson, Göteborg university, supported by Swedish Research council, 2017 – 2019 (Dnr 2016-00288)
- Longstanding collaboration with Xian Jiaotong university and the 2nd affiliated hospital in Xian, China.
- Cooperation with Fraunhofer research institute in Frankfurt Am Main.
- Guangdong province team project with Southern Medical university in Guangzhou, China
- Cooperation project with Prof. Gregg Fields, Florida Atlantic University, funded by NIH 2021-2023 (KR-K210)
- Cooperation with BioInvent international AB, Lund
- Cooperation with Sichuan University, West China Hospital in Chendu, China
Former and current PhD students
Awarded PhDs
Main supervision
- Mikael Andersson: 901214, "Self reactivity and tolerance in the immune system. Studies on T cells in murine type II collagen-induced arthritis", Medical Faculty, Uppsala University, opponent Antonio Coutinho, Paris.
- Tom J Goldschmidt: 910927, “T lymphocytes in autoimmune arthritis. Monoclonal antibody treatments in mouse and rat”, Medical Faculty, Uppsala University, opponent Anne Cooke, Cambridge.
- Liselotte Jansson, 940330; “Sex mediated influence in experimental models for rheumatoid arthritis and multiple sclerosis” Medical Faculty, Uppsala University, opponent Paul Wooley, Detroit.
- John A Mo, 941007; “B cell recognition of type II collagen in mouse cartilage” Medical Faculty, Uppsala University opponent Dan Holmberg, Umeå.
- Erik Michaëlsson, 960404, “T cell recognition of type II collagen - A cartilage glycoprotein of importance for autoimmune arthritis”, Medical Faculty, Lund University, opponent Polly Matzinger, New York.
- Vivianne Malmström, 970920, “Arthritis susceptibility and tolerance in collagen transgenic mice”, Medical Faculty, Lund University, opponent Erna Möller, Stockholm.
- Carina Vingsbo-Lundberg, 971108, “Chronic autoimmune arthritis in rats; pathogenesis and genetic factors”, Medical Faculty, Lund University, opponent Frank Emmrich, Leipzig.
- Ulrica Brunsberg, 980516, "The importance of major histocompatibility complex class II genes for the development of autoimmune inflammation", Medical Faculty, Lund University, opponent Ulf Gyllensten, Uppsala.
- Peter Kjellén, 991201, "The structural basis of MHC control in experimental autoimmunity", Medical Faculty, Lund University, opponent Sören Buus, Copenhagen.
- Alexandre Corthay, 000310, “T cells in autoimmunity, studies on murine type II collagen induced arthritis”, Medical faculty, Lund University, opponent George Kollias, Athens.
- Johan Jirholt, 001215, “A search for genes influencing autoimmunity. Focus on rheumatoid arthritis and multiple sclerosis”, Medical faculty, Lund University, opponent Holger Luthman, Stockholm.
- Mikael Vestberg, 010126, “MHC and transgenic mice. A study into function and polymorphism of class I and class II molecules”, Medical faculty, Lund University, opponent Jan Böhme, Stockholm.
- Ann-Sofie Hansson, 011019, “New models for relapsing polychondritis and rheumatoid arthritis, focus on the tissue specific response”, Medical faculty, Lund University, opponent Norman Staines, London.
- Åsa Johansson, 011019, “T cells, cytokines, and genetic factors in autoimmunity. Studies of experimental models of arthritis and sialadenitis”, Medical faculty, Lund University, opponent Linda Wicker, Cambridge.
- Shemin Lu, 020212, “Genetic analyses and vaccination of rat models of arthritis”, Medical faculty, Lund University, opponent Raimund Kinne, Jena.
- Lars Svensson, 020213, “Role of B cells and cytokines in arthritis”, Medical faculty, Lund University, opponent Hans Carlsten, Göteborg.
- Johan Bäcklund, 020924, “Studies on T cells reactive with posttranslational modification in arthritis”, Medical faculty, Lund University, opponent Ludvig Sollid, Oslo.
- Patrik Wernhoff, 020924, “Genetic control of autoantibodies in experimental autoimmune disease”, Medical faculty, Lund University, opponent Claudia Berek, Berlin.
- Peter Olofsson, 030527, “Identification of arthritis regulating genes in rats”Medical faculty, Lund University, opponent Tim Aitman, London.
- Jens Holmberg, 041210, “Reduced ROS production triggers arthritis”Medical faculty, Lund University, opponent Cor Verweij, Amsterdam.
- Martina Johannesson, 050128, “Novel strategies to explore the complex genetics of autoimmune diseases”Medical faculty, Lund University, opponent Karl Broman, Baltimore, USA.
- Robert Bockermann, 050523 “The role of T cells and T cell receptor polymorphism in controlling pathways leading to arthritis”Medical faculty, Lund University, opponent Juan Lafaille, New York, USA.
- Estelle Bajtner, 050917, “Role of collagen type II specific antibodies in arthritis”. Medical faculty, Lund University, opponent Marie Wahren, KI, Stockholm.
- Stefan Carlsen, 051015, “Cartilage proteins and their antigenic properties in arthritis”, Medical faculty, Lund University, opponent Richard Williams, London, UK.
- Kutty Selva Nandakumar, 060127, “Analysis of effector pathways in arthritis”, Medical faculty, Lund University, opponent Birgitta Heyman, Uppsala, Sweden
- Lina Olsson, 071130, “Genetic and genomic analysis of arthritis regulating regions in human and mouse.” Medical faculty, Lund University, opponent Janna Saarela, National Public Health Institute, Helsinki, Finland.
- Emma Ahlqvist, 071201, “Identification of arthritis susceptibility genes in mice and humans” Medical faculty, Lund University, opponent Jonathan Flint, Oxford University, Oxford, UK.
- Malin Hultqvist, 071214, “The role of reactive oxygen species in animal models of autoimmunity” Medical faculty, Lund University, opponent Philip Hawkins, The Babraham Institute, Cambridge, UK.
- Carola Rintisch, 090213, "From Disease to the Gene-identification of arthritis-regulating loci in rats" Medical faculty, Lund University, opponent Abdelhadi Saoudi, National Institute of Health and Medical Research, University Paul Sabatier, Hospital Purpan, Toulouse, France
- Therese Lindvall, 091127, "From Disease to Genes in Animal Models of Rheumatoid Arthritis and Multiple Sclerosis" Medical faculty, Lund University, opponent Krisitna Lejon, Immunology, Umeå University
- Angela Pizzolla, 111209, "The role of Macrophages in Regulating Inflammation by Oxidative Burst". Karolinska Institutet, opponent Kristoffer Hellstrand, Gothenburg University
- Michael Förster, 121027, "The Genetics of Experimental Arthritis in Rodents", Karolinska Institutet, opponent Göran Andersson, Swedish University of Agricultural Sciences, Uppsala
- Bruno Raposo, 121130, "Genetic Regulation of Autoantibodies in Arthritis: Lessons From Mouse Models". Karolinska Institutet, opponent Dan Holmberg, Copenhagen University, Denmark
- Ingrid Lindh, 130412, "Autoantibody Recognition of Collagen Type II in Arthritis". Karolinska Institutet, opponent Leendert Trouw, Leiden University, Netherlands
- Tiina Kelkka, 130426, "Reactive oxygen species in inflammation", University of Turku, opponent Lars Rönnblom, Uppsala University, Sweden
- Jonatan Tuncel, 130601, "Functional and Genetic Analyses of the MHC and its impact on Autoimmunity in the Rat", Karolinska Institute, opponent Jacques Neefjes, Netherlands Cancer Institute, Netherlands
- Sabrina Haag, 140412, "The MHC and the Recognition of Self and Altered Self in Experimental and Rheumatoid Arthritis" Karolinska Institute, Lars Fugger, University of Oxford, UK
- Anthony Yau 161209 “Positional identification and functional analysis of genes regulating autoimmune arthritis” Karolinska Institutet, opponent Prof Edward Wakeland, Dallas.
- Ulrika Norin 170324 "Identification of genetic variants and their implications in autoimmunity", opponent Marie Malissen, från Centre d’Immunologic de Marseille-Luminy France.
- Cecilia Hagert 171202 “The innate immune system as a driver of Rheumatoid Arthritis”, opponent Prof. Robert Harris, Karolinska Institutet
- Daniëlle Vaartjes 180616 “From genes to function in autoimmunity - a salty story” Karolinska Institutet, opponent Bryce Binstadt, Univ. of Minnesota
- Erik Lönnblom 210610 “Antibodies as pathogenic factors and biomarkers in rheumatoid arthritis”, opponent Prof. Carl Turesson, Lund University
- Gonzalo Fernandez Lahore 210820 “Identifying genetic determinants of T cell-dependent autoimmunity using forward genetics", Opponent: Prof. Vijay Kuchroo, Harvard Medical School, USA
- Jaime James 210924 “Redox Regulation of T cells in autoimmunity”, Opponent: Prof. Tomas Mustelin, University of Washington, USA
- Weiwei Cai 211105 ”Analysis of Antibodies to Cartilage Oligomeric matrix Protein in Rheumatoid Arthritis and in mouse Models”, Opponent: Prof. Silke Appel, University of Bergen, Norway
- Yanpeng Li 220524 “Human NCF1-339 polymorphism regulates JAK-STAT pathway through reactive oxygen species in autoimmune diseases”, Southern Medical University, China
- Alexander Krämer 230526 “Characterisation of epitope-specfic B cells and the role of IgG Fc sialylation in murine arthritis models”, Opponent Prof. Lars Nitschke, University of Erlangen, Germany
- Huqiao Luo 230605 “Unveiling the protective mechanisms of NOX2-derived ROS against autoimmune diseases”, Opponent Prof. Ann-Sophie Korganow, Strasbourg University Hospital, France
- Ana do Carmo Oliveira Coelho 230606 “Oxidative regulation of NCF1 in B cells and mouse models of arthritis”, Opponent Prof. Hans Martin Jäck, University of Erlangen, Germany
- Yibo He 230615 “Identification and functional analysis of anti-citrullinated protein antibodies in rheumatoid arthritis”, Opponent Associate Prof. Felipe Andrade, Johns Hopkins University, USA
- Taotao Li 230616 “Functional studies of anti-gpi monoclonal antibodies and anti-citrullinated protein antibodies (ACPAs)”, Opponent Prof. Thomas Pap, University of Münster, Germany
- Zhongwei Xu 231121 “Beyond conventional care: developing novel therapeutic approaches to combat arthritis”, opponent: Caroline Ospelt, University Hospital Zurich, Switzerland
- Alex Moreno Giro 240913 “Functional characterization of protective antibodies in murine models of rheumatoid arthritis”, opponent: Gestur Vidarsson, Utrecht University, the Netherlands
Current PhD students (as a main supervisor)
54. Michael Bonner (KI),
55. Carolin Svensson (KI)
Assistant supervisor for the following PhD
1) Per Larsson, UU 1989, main supervisor Lars Klareskog, 2) Inga Hansson, UU 1993, main supervisor Ragnar Mattsson, 5) Jinan Li, UmeåU 2004, main supervisor Tor Ny 3) Hai Tao Yang, UU 2000, main supervisor Prof. Ulf Pettersson; 4) Ingrid Teige, LU 2004, main supervisor Prof. Shoreh Issasadeh; 6) Meirav Holmdahl, LU 2005, main supervisor Prof. Anders Grubb 7) Jenny Karlsson, LU 2005, main supervisor Prof. Shoreh Issasadeh, 8) Alexandra Treschow, LU 2005, main supervisor Prof. Shoreh Issasadeh 9) Patrick Merky, KI 2011, main supervisor Docent Johan Bäcklund; 11) Ia Khalmadze, KI 2014, main supervisor Prof. Kutty Selva Nandakumar; 12) Simon Guerad, KI 2016 main supervisor Dr Kajsa Wing:; 13) Katrin Klocke, KI 2017, main supervisor Dr Kajsa Wing; 14) Bibo Liang, SMU 2017, main supervisor Prof. Zhao Ming; 15) Dongmei Tong, SMU 2018, main supervisor Prof. Zhao Ming, 16) Mike Aoun, Main supervisor Dr Liselotte Bäckdahl, 210903 “Positional cloning of polymorphic loci that control autoreactive T cells”, Opponent: Prof. Marc K. Jenkins, University of Minnesota, USA






