- >
- >
- Translational Molecular Imaging – Nordberg Lab
- NEUROPLASTICITY – DAGLIYAN GROUP
- Intracellular Mycobacterium Tuberculosis – Martin Rottenberg group
- Developmental and Translational Neurobiology – Cristiana Cruceanu's research group
- Microbiota–gut–brain axis and neurodevelopment – Rochellys Diaz Heijtz group
- Thoracic Surgery – Anders Franco-Cereceda's research group
- Electrophysiology – Mats Jensen-Urstad group
- Role of T cells in human host defense – Johan Sandberg group
- Germ cell biology and developmental programming in epigenetic inheritance of diseases – Qiaolin Deng's research group
- Unit for Bioentrepreneurship – Hanna Jansson's group
- Health informatics – Sabine Koch's group
- Cancer, rapid ageing and nutrition – Martin Bergö's research group
- Clinical radiation therapy – Åsa Carlsson Tedgren's Group
- TRANslational Theranostics Group – Thuy Tran
- Pathogenic pathways in Alzheimer Disease – Lars Tjernberg's research group
- Hematological and solid tumors – Research group Xu Dawei
- Developmental Neurogenomics – Michael Ratz's Group
- Notch signaling, ES cells, myogenic and vascular progenit breast cancer – Urban Lendahl's Group
- Particle toxicology – Hanna Karlsson's group
- Mitochondrial dysfunction in Alzheimer Disease – Maria Ankarcrona's research group
- Molecular pathology of the lung and pleura – Katalin Dobra's Group
- Late effects and cancer survivorship after diagnosis and treatment of aggressive lymphoma – Team Sandra Eloranta
- Research Group Anna Nopp
- Coding and non-coding RNAs in cancer – Per Hydbring's Team
- Research group Mikael Björnstedt
- Sister chromatid cohesion in DNA damage responses, DSB repair, Genome Integrity, Gene regulation – Lena Ström's Group
- Inborn Errors of Endocrinology and Metabolism – Anna Wedell's research group
- Functional Precision Medicine – Brinton Seashore-Ludlow's Team
- Understand skin using modern biology – Maria Kasper's Group
- Integrative Epidemiology – Fang Fang's research group
- Molecular Biometry – Roman Zubarev group
- Idiopathic pulmonary fibrosis (IPF) – Research group Magnus Sköld
- Molecular Neuroscience – Carlos Ibáñez group
- Research groups in psychology
- Research groups in forensic science
- Research groups in medical genetics and genomics
- Research groups in nursing
- Research groups in rheumatology and autoimmunity
- Genetic epidemiology of prostate and testicular cancer – Fredrik Wiklund's research group
- Systems biology of aging – Juulia Jylhävä's research group
- Aging – basic mechanisms and interventions – Christian Riedel's research group
- Embryonal, foetal and brain development – Juha Kere's research group
- Research groups in occupational therapy
- Growth and Cartilage Biology – Ola Nilsson's research group
- Infections and immunity in children with cancer – Anna Nilsson's research group
- Oxygenation, early resuscitation in trauma and drug treatment in cardiac arrest – Team Malin Jonsson Fagerlund
- Lars Karlsson team
- Sepsis and microcirculation – Sara Tehrani's research group
- Benson
- Visual Neuroscience – Rune Brautaset's research group
- Chemicals and female fertility – Pauliina Damdimopoulou Research Group
- Molecular and cellular exercise physiology – The Ruas Lab
- Social Gerontology – Neda Agahi group
- Neural circuits of cognition – Marie Carlén group
- Colorectal Surgery – Anna Martling and Caroline Nordenvall's research group
- Myeloma group – Evren Alici's research group
- Medicine and history of science – Eva Åhrén's group
- Cardiometabolic diseases – Ping Chen research group
- Anders Mutvei research group
- Ocular Oncology and Pathology – Gustav Stålhammar's research group
- Clinical Neurophysiology – Charith Cooray's research group
- Regulation of Gene Expression during Viral Infection – Gerald McInerney Group
- Social and affective learning and decision-making – Andreas Olsson's research group
- Translational Breast Cancer Research: Long-Term Risk and Endocrine Treatment Benefit – Linda Lindström's Group
- Notch3 and the cerebral small vessel disease CADASIL – from molecular mechanisms to treatment strategies – Helena Karlström's research group
- Dementia and Multimorbidity – Dorota Religa's research group
- Diagnostic Radiology – Lennart Nedar's research group
- Cognitive Neuropsychiatry – Predrag Petrovic's research group
- Chemical carcinogenesis – Ulla Stenius' group
- Metal toxicology – Maria Kippler's research group
- Cellular Stress in Health and Disease – Federico Pietrocola's group
- Autophagy and cardiovascular disease – Team Ewa Ehrenborg
- Research group Birgitta Sander & Birger Christensson
- Innate Immune Regulation – Quirin Hammer team
- Genetics of tumor predisposition and progression in breast cancer – Svetlana Bajalica Lagercrantz's Team
- Implementation and Quality (IMPAQT) – Claudia Hanson's research group
- Structural and Biophysical Immunology – Research group Adnane Achour
- Ulrika Marklund group
- Sustainable work & occupational safety and health – Emma Brulin's research group
- Health Systems Leadership, Management, and Safety – Carl Savage's group
- Cell and Molecular Immunology - Research group Cecilia Söderberg Naucler
- Brain Circuits – Konstantinos Meletis group
- Petter Ljungman's research group- Health effects of the Ambient Environment
- Neuropsychology of music – Fredrik Ullén group
- Research groups in geriatrics and gerontology
- Research groups in nutrition and dietetics
- Research groups in sports and fitness sciences
- Prostate Cancer – Henrik Grönberg's research group
- Methods of health promotion in the workplace – Lydia Kwak's research group
- Autism and perinatal epidemiology – Sven Sandin research group
- Wengström's research group
- Modelling and simulation with applications to cancer epidemiology and health economics – Mark Clements' research group
- Health Technology Assessment – Monica Hultcrantz’s group
- Pediatric anesthesiology Stockholm – Per-Arne Lönnqvist's research group
- Prehospital Emergency Care – Veronica Vicente's Research Group
- The brain after surgery and trauma - neuroinflammation, cognitive impairment and dementia – Lars I Eriksson research group
- Personalized diet and medications for cognition – Garcia-Ptacek group
- Perpetration Prevention – Rahm and Joleby's research group
- SOLIID - Sustainable Organizational Learning, Innovation, Improvement and Development in Health and Social services – Monica Nyström's group
- Social Gerontology – Carin Lennartsson group
- Genetic and molecular basis of nervous system disorders – Andrea Carmine Belin group
- Chronobiology – Petrus lab
- Leukemic stem cells – Petter Woll group
- Nanomedicine and Spatial Biology – Teixeira Lab
- Global Disaster Medicine - Health Needs and Response – Johan von Schreeb's research group
- Translational cardiac and skeletal muscle physiology – Daniel Andersson's research group
- Neuroradiology – Staffan Holmin's research group
- Tumour genomics – Lauri Aaltonen's research group
- Neurobiology of Motor Actions – Abdel El Manira group
- Neuroinflammation in Alzheimer disease – Marianne Schultzberg research group
- Palliative Medicin – Linda Björkhem-Bergman's research group
- Nucleotide metabolism and molecular pharmacology – Sean Rudd's Group
- Cardiovascular Inflammation – Research group Peder Olofsson
- Mesenchymal stem cells – Katarina Le Blanc group
- Luigi De Petris' Team
- Autoimmunity and cancer – Research group Marie Wahren-Herlenius
- Atherothrombosis research – Team Nailin Li
- Precision cancer medicine – Olli Kallioniemi's group
- Research group Stephan Mielke
- Genetics of autoimmune diseases – Team Leonid Padyukov
- Lifestyle, health and pain in musculoskeletal disorders – Nina Brodin's research group
- Vascular Functions in Metabolic and CNS Diseases – Ulf Eriksson group
- Sarcoma Genomics – Felix Haglund de Flon's Group
- Team Helene Alexanderson
- Injury and Repair in the Immature Brain – Blomgren group
- Statistical methods for cancer patient survival – Therese Andersson's research group
- Individual differences in cognition – Agneta Herlitz’ research group
- Research groups in health economy, healthcare management and policy
- Research groups in reproductive medicine and gynaecology
- Research groups in structural biology
- Research groups in artificial intelligence
- Work environment, health and productivity – Christina Björklund's research group
- Predictive medicine – Mattias Rantalainen's research group
- Epigenetic regulation of leukemia and normal blood development – Andreas Lennartsson's research group
- Epigenetic mechanisms underlying metabolic-inflammatory diseases – Eckardt Treuter's research group
- The IMPACT research group – Marie Löf
- Stockholm Center for Health Economics (StoCHE) – Emelie Heintz's team
- Towards personalized physiology in Intensive Care – Team Johan Mårtensson
- Biophysics – Erdinc Sezgin's Lab
- War, crisis, and security studies
- Systems Medicine – Johan Björkegren research group
- Research group Kilian Eyerich
- Global infections – Research group Anna Färnert
- Translational Microbiome Research and Pandemic Preparedness – Lars Engstrand group
- EcoMind and biological pathways in cognitive aging – Debora Rizzuto group
- Circuits controlling Action and their Evolution – Sten Grillner group
- Mikael Rydén & Niklas Mejhert lab
- Understand Fundamental Immune Mechanisms to Develop New Treatments for Lung Diseases – Tim Willinger group
- Clinical Brain Imaging Methods – Anna Falk Delgado's research group
- Hyperbaric medicine – Peter Lindholm's research group
- Eye movements and vision – Tony Pansell's research group
- Lung toxicology – Lena Palmberg's research group
- The NeuroCardioMetabol Group – Thomas Nyström & Cesare Patrone
- Environmental and genetic factors influence on neurodevelopment – Sandra Ceccatelli group
- Skin wound healing – Research group Ning Xu Landén
- From development to disease: decoding stem cell programs for CNS repair – Erik Sundström's and Xiaofei Li's research group – Erik Sundström-Xiaofei Li group
- Neurodegenerative diseases and Aging – Daniel Ferreira Research Group
- Deep learning models of cancer mechanisms – Avlant Nilsson's Group
- Clinical Physiology – Marcus Carlsson's research group
- Molly Stevens group
- Molecular Mechanisms of Viral Oncogenesis – Maria G. Masucci's Group
- Björn Reinius group
- Quantitative principles in tumour biology – Jean Hausser's Group
- Bone biology – Sara Windahl Research group
- Research group Volkan Özenci
- Genetics and mechanisms of metals – Karin Broberg's research group
- Immunodeficiency Diseases – Lisa Westerberg Group
- Structural studies of fertilisation and zona pellucida module proteins – Luca Jovine's research group
- Tissue regeneration, focusing on fat cells (adipocytes) and obesity – Kirsty Spalding's Group
- Robert Månsson group
- Developmental and Regenerative Stem Cell Medicine – Lanner Lab
- Suicide and mental health lab – Vladimir Carli and Gergö Hadlaczky's group
- Medical, genetic and molecular decoding of neurodevelopmental disorders – Kristiina Tammimies' research group
- Working hours, Recovery and Safety in working life. – Anna Dahlgren's research group
- Research groups in hematology
- SOMIND: Somatic, Mental, and Neurodevelopmental Conditions – Agnieszka Butwicka's research group
- Research groups in drug abuse and addiction
- Research groups in biomaterials science
- Mikael Adner's research group
- Chemical and functional neuroanatomy of pain and stress systems – Tomas Hökfelt group
- Spider silk biology for biomedical applications – Anna Rising's research group
- Cancer Epidemiology and Causal Inference – Research group Maria Feychting
- Protein biochemistry for development of novel medical treatments – Janne Johansson's research group
- Healthcare financing and organization – Clas Rehnberg's group
- Space and environmental physiology – Rodrigo Fernandez-Gonzalo group
- Ninib Baryawno research group
- Neurohormonal Basis of Physiological and Maladaptive Expressions of Survival Behaviors – Stefanos Stagkourakis group
- Breast cancer precision medicine – Marike Gabrielson's research group
- Atopic dermatitis – Research group Emma Johansson
- Reproductive endocrinology and metabolism – Angelica Lindén Hirschberg's research group
- Genetic basis for B and T cell recognition and function – Gunilla Karlsson Hedestam Group
- Cognitive Aging and Mental Health – Erika Jonsson Laukka Group
- Molecular and circuit neuropharmacology – Gilberto Fisone group
- The role of immunity in shaping adipose tissue development and metabolism – Jurga Laurencikiene group
- Chronic myeloid malignancies – Johanna Ungerstedt group
- Studies of tissue microbiology and immunology – Mattias Svensson group
- Sten Linnarsson group
- Balance, gait, exercise and physical activity in neurological diseases – Franzén Group
- Global Health Pharmacology and Therapeutics (GH-Pharma) – Eleni Aklillu's research group
- Real-world-data in Multiple Sclerosis – Anna Glaser's research group
- Suicidology and transcultural psychiatry – Marie Dahlin's research group
- Experimental and Clinical Neuroendocrinology – Maria Petersson's research group
- Cutting edge translational research of common and rare skin disease – Research group Jakob Wikström
- Alzheimer’s Disease Risk Factors & Sex-Specific Mechanisms – Silvia Maioli's research group
- Frontal lobes and cognitive failure – Wahlund's research group
- Clinical Pharmacology – Research group Rickard Malmström
- Molecular Vascular Medicine – Team Lars Maegdefessel
- Molecular endocrine pathology – Christofer Juhlin's Group
- Presynaptic mechanisms – Lennart Brodin group
- Malignant melanoma – Hildur Helgadottir's Group
- Precision strategies to eradicate heterogeneous tumor cells in pediatric cancer – Kasper Karlsson's Group
- Respiratory and systemic immune responses in human pulmonary viral infection and inflammation – Research group Anna Smed Sörensen
- Genetic toxicology – Kristian Dreij's group
- Identifying new molecular targets for the treatment of motor neuron diseases – Eva Hedlund's research
- Epigenetics - basic mechanisms and disease – Karl Ekwall's research group
- Forensic medicine – Henrik Druid's Group
- Ageing and Health – Karin Modig's research group
- Perception Neuroscience – Johan Lundström's research group
- Computational medicine and bioinformatics – Trung Nghia Vu's research group
- Toxicology – Bertrand Joseph´s Research group
- Etiology, prevention and treatment of eating disorders – Ata Ghaderi's research group
- Research groups in medical ethics and history of science
- Research groups in occupational and environmental health
- Research groups in surgery
- Research groups in biophysics
- Research Group Johan Ärnlöv
- Narrative in health and social care – Research group
- Cellular and molecular mechanisms underlying intervertebral disk formation and Diversity and plasticity of adipose tissue cell niche – Meng Xie's research team
- Neuroimmunology of cancer – Sébastien Talbot
- Pediatric Systems Immunology – Petter Brodin's research group
- Microvascular oxidative stress in the triad of cardiovascular, metabolic and renal disease – Mattias Carlström group
- Cardio-renal epidemiology – Juan-Jesus Carrero's research group
- Novel treatment approaches for neuroblastoma – Malin Wickström Näsman research group
- Cancer immune and gene therapy – Rolf Kiessling's Team
- Amanda Andersson Rolf's Group
- Movement, Activity and Health – Eva Broström's research group
- Multidimensional health in old age – Amaia Calderón-Larrañaga group
- Molecular epidemiology of aging – Sara Hägg's research group
- Long noncoding RNA in the adipocyte – Alastair Kerr Research Group
- Leukemia niche – Hong Qian group
- Disease mechanisms in severe acute bacterial infections – Anna Norrby-Teglund group
- Gastrointestinal pediatric surgery – Tomas Wester's research group
- Developmental biology and regenerative medicine – Igor Adameyko's research group
- Experimental audiology – Barbara Canlon's research group
- Translational Immunology in Neuroinflammation – Olivia Thomas' research group
- Sickness absence, health and living conditions – Emilie Friberg's research group
- Health Systems and Policy – Cecilia Stålsby Lundborg's research group
- Endocrinology and autoimmune diseases – Research group Sophie Bensing
- Health economics of Alzheimer disease and other dementias – Linus Jönsson Group
- Epidemiology: Diet, contaminants and health – Agneta Åkesson's research group
- Neuronal circuits of anxiety – Janos Fuzik group
- Paediatric rheumatology – Research group Helena Erlandsson Harris
- Cardiovascular Epidemiology – Karin Leander research group
- Molecular cardiology – Research group Francesco Cosentino
- Björn Högberg group
- Regeneration mechanisms – Andras Simon's Group
- Assessing and managing chemical risks – Linda Schenk's research group
- Autoimmunity and adaptive immunity in rheumatic disease – Research group Vivianne Malmström
- Rheumatic systemic diseases – Team Marie Holmqvist
- Heart failure with reduced and preserved ejection fraction. Clinical and translational aspects. – Research group Lars Lund
- Christoph Ziegenhain Group
- Psychiatric epidemiology – Paul Lichtenstein's research group
- Structural basis of RNA biogenesis – Martin Hällberg's research group
- Hematopoietic Stem Cell Biology Group – Sten Eirik W. Jacobsen Lab
- Psychiatric Genetic Epidemiology – Sarah Bergen's research group
- Renal Medicine at Novum (Baxter Novum) – Bengt Lindholm Group
- Research groups in biochemistry
- Psychological treatment: internet and exposure – Brjánn Ljótsson's research group
- Research groups in immunology
- Research groups in ophthalmology
- Research groups in urology and nephrology
- Research groups in sociology and migration
- Integrative physiology – Juleen Zierath and Anna Krook
- Hormonal signalling and cancer – Cecilia Williams research group
- Cell Biology of Cancer – Staffan Strömblad's research group
- Chronic inflammation with focus on rheumatology and cancer – Research group Per-Johan Jakobsson
- Health services and social work research – Lena Von Koch's research group
- Nitrate-nitrite-NO pathway in health and disease – Eddie Weitzberg's research group
- Human Translational Genetics – Stefano Romeo Group
- Clinical Pancreatology – Miroslav Vujasinovic research group
- Hemostasis and Thrombosis – Mika Skeppholms research group
- Amyotrophic Lateral Sclerosis - ALS – Caroline Ingre's research group
- Saving the brain in critically ill neonates – Ulrika Ådén's research group
- Regulatory Transcriptomics – Kutter group
- NeuroimAging – Grégoria Kalpouzos group
- Section of Pharmacogenetics – MIS-lab
- Biological and clinical aspects of the myelodysplastic syndrome – Magnus Tobiasson group
- Human innate lymphoid cell lab – Jenny Mjösberg group
- Viruses and their interactions with the immune system – Sara Gredmark Russ group
- Protein degradation pathways – Helin Norberg's research group
- Translational Auditory Neuroscience – Christopher Cederroth's research group
- Neuroradiology: Neurodegeneration and Neuroinflammation – Tobias Granberg's research group
- Tumor Immunology and Immunotherapy Group – Dhifaf Sarhan
- Causal inference in epidemiologic research – Arvid Sjölander's research group
- The role of autophagy in Aβ metabolism and neurodegeneration in Alzheimer’s disease – Per Nilsson group
- Darreh-Shori's Lab/group
- PROCOME – Hanna Öfverström's and Marta Roczniewska's group
- Molecular brain imaging of neurodegenerative disorders – Andrea Varrone's research group
- Alcohol and Drug Epidemiology – Mats Ramstedt's research group
- Anxiety related disoders and genetics – Christian Rück's research group
- Psychotherapy Research – Tobias Lundgren's research group
- Human olfaction – Mats J. Olsson's research group
- Tissue stem cells and aging – Pekka Katajisto's Group
- SOSVASC – David Lindström's research group
- Yenan Bryceson group
- Epidemiology of sarcoidosis and systemic lupus erythematosus – Team Elizabeth Arkema
- Viral genomics and metagenomics – Tobias Allander Group
- Defining the metabolic signatures of tissue resident human ILCs – Chris Tibbitt team
- Human papillomavirus which causes cervical cancer – Research Group Karin Sundström
- Research groups in bioinformatics and systems biology
- Cognitive epidemiology – Bo Melin's research group
- Gilad Silberberg group
- Research groups in oto-rhino-laryngology and logopaedics
- Immunoregulation of environmental-induced airway inflammation in severe asthma and bronchiectasis – Apostolos Bossios's research group
- Multimorbidity and frailty in older adults – Davide Liborio Vetrano group
- Psychiatric and pharmaco-epidemiology – Zheng Chang's research group
- Charting normal and malignant hematopoiesis – Team Joakim Dahlin
- Molecular mechanisms governing oxygen homeostasis in development, evolution, and disease – Kragesteen's research group
- Precision Medicine – Infrastructure team
- Research groups in medical epigenetics and epigenomics
- Medical sensors and diagnostics – Team Onur Parlak
- Uncovering the molecular and physical principles governing early embryonic division and nuclear organization – Jan Ellenberg Group
- Nanoscale lipid flux architecture – Veijo Salo’s Research Group
- Pediatric and respiratory epidemiology – Catarina Almqvist Malmros' research group
- Sexual and Reproductive Health – Marie Klingberg-Allvin research group
- Angiogenesis, Cancer, Metabolic disease, Cardiovascular disease, Eye disease – Yihai Cao Group
- Cardiovascular and psychosocial health in resilience and healthy brain aging – Chengxuan Qiu Group
- Translational Psychiatry – Catharina Lavebratt's research group
- Rare metabolic liver diseases, coagulation and hepatocellular carcinoma – Stål and Wahlin research group
- Blood Engineering – Vanessa Lundin group
- Development, regulation and function of human innate lymphoid cells throughout life – Jakob Michaëlsson group
- Clinical Genetics – Richard Rosenquist Brandell's research group
- Oxysterols – Ingemar Björkhem and Ulf Diczfalusy research
- Molecular exercise physiology – Carl Johan Sundberg's research group
- Large-scale Network Connectivity in the Human Brain – Peter Fransson's research group
- Environmental physiology
- The Systems Virology Lab – Ujjwal Neogi
- Cancer Cell Invasion – Marco Gerling's research group
- Molecular brain imaging, neuropsychopharmacology, depression and anxiety disorders – Johan Lundberg's research group
- Organization and operation of postural neuronal networks in health and disease – Tatiana Deliagina group
- Paediatrics – Anna Lindholm Olinder's research group
- Petzold group
- Molecular and epidemiological studies of ascending aortic aneurysms – Research group Hanna Björck
- Prevention, Intervention and Mechanisms in Public Health (PRIME Health) – Cecilia Magnusson's research group
- The interplay between circadian 3D genome organization and metabolism in complex diseases – Anita Göndör's Group
- Reconstructive Plastic Surgery and Global Surgery – Jenny Löfgren's research group
- Real-world effectiveness of psychopharmacological treatment – Jari Tiihonen's Research Group
- Center for Heart Failure and Arrhythmia – Gianluigi Savarese
- Sports Medicine – Anders Stålman's research group
- Women's Mental Health Epidemiology – Donghao Lu Lab
- The SMC complexes, chromosome dynamics and stability – Camilla Björkegren's Group
- Extracellular vesicles in immunity – Research group Susanne Gabrielsson
- Precision Cancer Medicine in Lung Cancer - Preclinical, Translational & Clinical Research – Simon Ekman's research group
- RNA-guided DNA repair and cancer – Marianne Farnebo's research group
- Laboratory of translational fertility preservation – Kenny Rodriguez-Wallberg's group
- Research groups in cancer and oncology
- Common Mental Disorders and Behavioral Medicine in Primary Care – Erik Hedman's research group
- Neuronal membrane trafficking – Oleg Shupliakov group
- Research groups in paediatrics
- Biostatistics With Focus on Statistical Methods in Cancer Epidemiology and Screening – Keith Humphreys' research group
- Research groups in nanotechnology
- Gastroenterology – clinical epidemiology and clinical trials – Research group Ola Olén
- Precision engineering of T cells in vivo for therapies of cancer – William Nyberg team
- Protein Misfolding and Prevention by Molecular Chaperones in Cancer and Neurodegenerative Disease – Gefei Chen's research group
- Social Perception – Arvid Guterstam's research group
- Neuroepidemiology – Kyla McKay and Katarina Fink's research group
- Experimental growth research – Lars Sävendahl's research group
- Spinal circuits for whole-body motor coordination – Laurence Picton group
- Clinical HIV epidemiology and comorbidity – Christina Carlander Research group
- Head and neck pathology – Anders Näsman's Team
- Elias Arnér research group
- Gynecologic Cancer – Henrik Falconer's research group
- B cell biology, from immunodeficiency to cancer – Pan Hammarström Lab
- Developmental Cognitive Neuroscience – Torkel Klingberg group
- Rare Diseases – Ann Nordgren och Anna Lindstrand's research group
- Research on steatotic liver disease – Hagström Group
- Stem Cell Size – Jette Lengefeld Team
- Natural Killer Cell Biology and Cell Therapy – Kalle Malmberg group
- Mast Cell Biology – Research group Gunnar Nilsson
- Traumatic Brain Injuries and Neuromonitoring – Eric Thelin's research group
- Medical Statistics team
- Augmented Neurosurgery – Erik Edström and Adrian Elmi Terander's research group
- Molecular neurophysiology – Karima Chergui's research group
- Injuries Social Aetiology and Consequences (ISAC) – Lucie Laflamme's research group
- Unconventional T cells in human pathology – Andrea Ponzetta team
- Inflammation and immune regulation during infection – Team Christopher Sundling
- Orthopaedics – Anders Enocson's research group
- Endothelial dysfunction in atherosclerotic cardiovascular disease – Research Group John Pernow
- Clinical epidemiology of multiple sclerosis and inflammatory joint diseases – Team Thomas Frisell
- Cancer stem cells and clonal structure in acute lymphoblastic leukemia – Martin Enge's Group
- Mitochondrial metabolism in health and disease – Anna Wredenberg group
- Biomolecular Medicine and Advanced therapies- focus on delivery of RNA therapeutics – Research group Samir EL Andaloussi
- Antimicrobial resistance – Christian Giske research group
- Tissue immunology – Research group Helen Kaipe
- Health Economics and Economic Evaluation – Niklas Zethraeus' group
- Mental Health Neural Dynamics Lab – Kristoffer Månsson's research group
- Stem Cell Biology – Jonas Muhr's Group
- VIVAC research group: Vaccines and Immunotherapies against Viruses And Cancer – Matti Sällberg
- The passage of the mRNP particle through the nuclear pore – Bertil Daneholt's research
- Pharmacoepidemiology – Team Björn Pasternak
- Therapeutic Immunology and Transfusion Medicine – Michael Uhlin’s research group
- Musculoskeletal conditions and sports medicine research – Iben Axén's research group
- Research groups in cancer and oncology
- Nutritional neuroscience – Janina Seubert's Research Group
- Tobias Karlsson's group
- Research groups in pharmacology and toxicology
- Precision Psychiatry – Lu Yi's research group
- Research groups in medicinal chemistry
- Agneta Richter-Dahlfors group
- Cancer epidemiology with focus on breast cancer and applied biostatistics – Anna Johansson's research group
- Autism, ADHD and other neurodevelopmental conditions – Sven Bölte's research group
- Twinstudies on health conditions and sickness absence – Pia Svedberg's research group
- Psychiatric comorbidity and intergenerational transmission of mental health problems – Erik Pettersson's research group
- Neuronal circuits of the Basal Ganglia – Maya Ketzef group
- Psychological Interventions - innovation, improvement and implementation – Viktor Kaldo's research group
- Susanna Ranta team
- Neurovascular diseases – Christina Sjöstrand research group
- Gonçalo Castelo-Branco Group
- Pediatric Neuroimmunology and Epilepsy – Ronny Wickström's research group
- Explores various cell signaling phenomena and their impact on critical biological and medical processes using advanced light microscopy – Per Uhlén Group
- Neural stem cells – Anna Falk group
- Diabetes and Associated Complications – Sergiu-Bogdan Catrina's research group
- Myelodysplastic syndromes – Eva Hellström Lindberg group
- Immune responses to human viral infections and cancer – Hans-Gustaf Ljunggren group
- Health services research in neurological conditions – Charlotte Ytterberg's research group
- Neurosurgery – Mikael A. Svensson's research group
- Westman neuroimaging group
- Stemcells and Inflammation – Lou Brundin's research group
- Pharmacological nitric oxide research – Jon Lundberg's research group
- Epidemiology of Psychiatric Conditions, Substance use and Social Environment (EPiCSS) – Emilie Agardh's & Renee Gardner's Research Group
- Autoimmune neurology – Jakob Theorell team
- Reducing antibiotic resistance – Research group Pontus Nauclér
- Orthopaedics – Karl Eriksson's research group
- Endocrine Surgery – Robert Bränström's research group
- Paediatrics – Inger Kull's research team
- Cancer proteogenomics - from methods to clinical applications – Janne Lehtiö's Group
- Primary Immunodeficiency, Innate Immunity and Antimicrobial peptides – Peter Bergman Research Group/The AMP-group
- Clinical Virology and Immunology – Annika Karlsson's research group
- Jussi Taipale Group
- Global Child Health and the Sustainable Development Goals – Tobias Alfvén's research group
- Topoisomerases, chromatin biology and cancer – Laura Baranello's group
- Stem cells and neural development – Johan Ericson's Group
- Vascular morphogenesis and function in health and disease – Lars Jakobsson group
- Epidemiology and Public Health Intervention Research (EPHIR) – Jette Möller's research group
- Epithelial stem cells in development and disease – Maria Genander's group
- Research Group Gustaf Edgren
- Drug discovery, pancreatic beta-cell regeneration – Olov Andersson's Group
- Unit of Biostatistics – Matteo Bottai
- Research groups in cardiology and cardiovascular diseases
- Sleep, cognition and health – John Axelsson's research group
- Research groups in infectious medicine
- Research groups in physiology and anatomy
- Cardiometabolic epidemiology and aging – Ida Karlsson's research group
- How the overview of research subjects is made
- Precision Cancer Control Group – Martin Eklund's research group
- Liver and monocyte remodelling in non-alcoholic fatty liver disease and cardiovascular disease – Rongrong Fan's research group
- Early childhood development and neurodevelopmental conditions – Terje Falck-Ytter's research group
- Mental health and social integration (Mente) – Ellenor Mittendorfer-Rutz's research group
- Mechanisms of protein aggregation and inhibition – Axel Abelein group
- Genetic and pharmacological epidemiology – Zeberg laboratory
- REACH – digital treatment and prevention – David Moulaee Conradsson's research group
- Mucosal immunology lab – Charlotte Thålin research group
- Stroke – Annika Lundströms research group
- Environment, nutrition and health – Anna Bergström's research group
- Genetic and epigenetic factors in asthma and allergy – Cilla Söderhäll's research group
- Cardiovascular epidemiology – Team Bruna Gigante
- Neurobiology of Stress and Treatment Response – Juan Pablo Lopez group
- Vascular Surgery – Ulf Hedin's research group
- Annika Bergquist group
- Acute myeloid leukemia – Sören Lehmann group
- Human tissue-resident NK cells – Niklas Björkström group
- Neuropsychoimmunology – Sophie Erhardt's research group
- Diabetes epidemiology – Sofia Carlsson's research group
- Stroke - Acute Intervention and Secondary Prevention – Niaz Ahmed's research group
- Molecular muscle physiology and pathophysiology – Lanner Lab
- Community nutrition and physical activity (CoNPA) – Liselotte Schäfer Elinder's research group
- Inflammatory bowel disease (IBD) – Research group Eduardo Villablanca
- CRISPR-based drug target discovery in cancer and autoimmunity – Research group Fredrik Wermeling
- Emergency care – Kristian Ängeby's research group
- Clinical Cancer Epidemiology – Research group Karin Ekström Smedby
- Cancer prevention and screening – Johannes Blom's research group
- Somatosensation – Patrik Ernfors group
- DNA replication & Cancer Genetics – Lemmens Group
- Laboratory testing for alcohol and drugs of abuse – Anders Helander research
- Anaesthesia and Intensive care – Rebecka Rubenson Wahlin/Anna Schandl's research group
- Jonas Fuxe Group
- SCF ubiquitin ligases, cell cycle, transcription and cancer development – Olle Sangfelt's Group
- Toxicological Mechanisms – Emma Wincent's research group
- Stem Cells in Tissue Homeostasis and Regenerative Medicine – Jonas Frisén's Group
- Urology – Olof Akre's research group
- The New World of Work – Theo Bodin's research group
- Midbrain dopaminergic neuron development
- Research group Michael Fored
- Neurobiology of pain & Therapeutics – Saida Hadjab Group
- Risk assessment – Mattias Öberg's research group
- Research groups in cell and molecular biology
- Developmental Psychology: Digital Media and ADHD – Lisa Thorell's research group
- Research groups in education and pedagogy
- Research groups in biostatistics and probability theory
- ESSI – Emotion regulation, Self-injury, Suicide, and Intervention – Johan Bjureberg's research group
- Medical ethics – Gert Helgesson's group
- Treatment of substance use disorders – Johan Franck's Research Group
- Sex hormones and sex differences in diseases of the brain – Ivan Nalvarte's research group
- Cilia in the brain – and their connections to human brain disorders – Peter Swoboda's research group
- Evidence-based methods in autism and ADHD – Tatja Hirvikoski's research group
- Long-Term Outcomes after Perioperative and Intensive Care – Max Bell research group
- Accelerating drug discovery using molecular modeling and machine learning – Andreas Luttens' group
- Pediatric Healthcare Science – Cecilia Bartholdson research group
- Digital Strategies for AF Detection and Outcome Prevention – Emma Svennberg Team
- Translational Pharmacology – Kent Jardemark's team
- Global and Sexual Health (GloSH) – Anna Mia Ekström's research group
- Medical Inflammation Research – Rikard Holmdahl group
- Neuro-infections & Neuroinflammation – Federico Iovino Group
- Clinical Chemistry and Blood Coagulation – Jovan Antovic's research group
- Translational research on human microbial infections and its consequences – Anders Sönnerborg's group
- Human tissue-resident NK cells in homeostasis and disease – Nicole Marquardt team
- Etiology and pathogenesis of type 1 diabetes – Malin Flodström-Tullberg group
- Oxygen sensing, cancer and intratumor heterogeneity – Schlisio Lab
- Genetic Epidemiology of Neuroinflammatory Disorders – Ingrid Kockum's research group
- Retina – Anders Kvanta's research group
- Glaucoma – Pete Williams' research group
- Leadership in healthcare and academia – Mia von Knorring's group
- Luminal gastroenterology – Research group Charlotte Hedin
- Identifying molecular signals in the genital mucosa that determine susceptibility to sexually transmitted infections – Research group Kristina Broliden
- Coronary heart disease – Research group Per Svensson
- Educational development – Matti Nikkola's group
- Post-transcriptional regulation in mitochondria – Joanna Rorbach group
- Cellular diversity is generated during development, neuronal identity – Johan Holmberg's Group
- Lipid handling in health and cardiometabolic disease – Research group Carolina Hagberg
- Renal glomerulus biology and diseases – Jaakko Patrakka group
- Research group Caroline Palm Apergi
- Precision pathology and tumor heterogeneity – Johan Hartman's Group
- Lifelong Learning in Health Care Contexts – Terese Stenfors' group
- Translational Arthritis Research – Team Bence Réthi
- Stem cells, Developmental Biology, Heart Disease, Heart Development, Genetics, Biotechnology – Kenneth R. Chien's group
- Urological cancer – Lars Egevad's Group
- The ubiquitin/proteasome system in neurodegenerative diseases and cancer – Nico Dantuma's Group
- Experimental alcohol- and drug dependence research – Vladana Vukojević research group
- Chronic inflammatory disease epidemiology – Research group Johan Askling
- Statistical and bioinformatics analyses of high-throughput molecular data – Yudi Pawitan's research group
- Urban environment and children´s and youth health – Olena Gruzieva's research group
- Research groups in laboratory medicine
- Research groups in medical biotechnology
- Research groups in physiotherapy
- Breast cancer epidemiology – Kamila Czene's research group
- Research groups in human computer interaction
- Research group Liv Eidsmo
- Preventive Medicine – Susanna Larsson's research group
- Transition to adulthood for individuals with neurodevelopmental conditions – Ulf Jonsson's team
- Function and Health in Respiratory and Cardiovascular Conditions – Malin Nygren-Bonnier's research group
- Laboratory of lymphocyte biology – Team Taras Kreslavskiy
- Translational and clinical research in acute myeloid leukemia – Team Martin Jädersten
- CEREBRA – Susanne Palmcrantz's research group
- Speech-Language Pathology – Liza Bergström's research group
- Tissue immunosurveillance by cytotoxic lymphocytes – Takuya Sekine team
- Physical activity and Sports medicine with focus on prevention – Hagströmer research group
- Receptor biology and signaling – Schulte Lab
- Neurobiology of Obesity – Alessandro Furlan group
- Upper GI Surgery – Jesper Lagergren's research group
- Sepsis and COVID-19 – Kristoffer Strålin Group
- Harnessing Neutrophils for Precision Cell Therapy against solid tumors – Roland Fiskesund Team
- Human T cell immunity to evolving viruses and cancers – Marcus Buggert group
- Neuroradiology - MRI physics – Stefan Skare's research group
- Wilhelm lab
- Inflammatory responses in cancer and autoimmunity – Mikael Karlsson's Group
- Cultural Medicine – Solvig Ekblad's unit
- Working life, ergonomics, psychosocial factors, and health – Research group Daniel Falkstedt
- Aging and health – Welmer's research group
- NK Cell Recognition – Klas Kärre Group
- Computational Breast Imaging – Fredrik Strand's Group
- Drug treatment – Erik Eliasson research group
- Neural differentiation as a strategy for neuroblastoma treatment – Marie Arsenian Henriksson Group
- Cancer immunotherapy – Mattias Carlsten group
- Cell-based immune therapy for cancer – Andreas Lundqvist's Group
- Basic molecular structure of human skin – Lars Norlén's research
- Reproductive, Perinatal and Pediatric Epidemiology – Research group Olof Stephansson
- Research group Erik Melén
- Research group Joel Nordin
- MINT – Klas Karlgren's team
- Translational breast cancer research – Theodoros Foukakis' Group
- Genes function and molecular bases of diseases – Research group Magdalena Paolino
- Homeostasis and tissue repair mechanisms in the mammalian system, pericytes, stem cells – Christian Göritz Group
- The Perinatal Epidemiology Lifecourse Lab – Team Neda Razaz
- Experimental Cancer Medicine (ECM)
- Research group Therese Djärv
- Molecular and cellular neuroendocrinology – Tibor Harkany group
- Neuropsychiatric disorders – Håkan Karlsson group
- Research groups in dermatology and venereal diseases
- Evidence-based practice: prevention, intervention and implementation – Pia Enebrink's research group
- Research groups in radiology and medical imaging
- Research groups in psychiatry
- Maria Eriksdotter research group
- Research groups in precision medicine
- Breast cancer surgery – Jana de Boniface's research group
- ICare – Ann Langius-Eklöf's research group
- Immunology and Chronic Disease – Johan Frostegård's research group
- Health in Everyday Life (HELD) – Susanne Guidetti's research group
- Center for Resuscitation Science – Jacob Hollenberg's research group
- Applied Developmental Neurobiology – Carl Sellgren research group
- Global Reproductive Outreach & Wellness (GROW) – Michael Wells
- Mats Marshall Heyman research group
- SLE/APS/Vasculitis group – Research group Aleksandra Antovic
- Ute Römling group
- ALGOSH: Algorithmic Management at Work
- Development of autonomic control – Herlenius Research
- Maintenance and expression of mtDNA in disease and ageing – Nils-Göran Larsson Group
- Neuropharmacology - Movement Disorders – Per Svenningsson's research group
- Somatosensation & Gargalesis – Konstantina Kilteni group
- Signal Transduction – Ismael Valladolid Acebes' research group
- Microbiome and Infections – Piotr Nowak Research Group
- Immunological tolerance and transfusion immunology – Petter Höglund group
- Immunopathogenesis and new therapeutic strategies in tuberculosis – Susanna Brighenti group
- Pain and Brain Imaging – Karin Jensen's research group
- Susanne Nylén group
- Hand surgery – Maria Wilckes Research group
- Environmental impact on pulmonary host defence and chronic airflow obstruction – Anders Lindén's research group
- Rehabilitation, collaboration and aging – Elisabeth Rydwik's research group
- Tuberculosis Aerobiology – Antonio Rothfuchs group
- Oscar Fernandez-Capetillo group
- Diversity within the T cell response, T cell memory – Research group Carmen Gerlach
- Clinical and translational melanoma research – Hanna Eriksson's Group
- Meiotic chromosome segregation – Christer Höög's research group
- B cells and autoantibodies in rheumatic disease – Team Caroline Grönwall
- Precision medicine in lymphoid malignancies including CLL; from new targets to real-world assessments – Anders Österborg's Group
- Research group for Cancer evolution
- Jiri Bartek group
- Translational Cardiology (@TransCardio) – Research group Magnus Bäck
- Gene regulation, genome-wide experimental and computational techniques – Rickard Sandberg's Group
- The Helleday Laboratory focuses on harnessing defects in the DNA damage response and metabolism to develop novel therapies
- Small RNAs in cancer development – Weng-Onn Lui's Team
- Geriatric pharmacoepidemiology – Kristina Johnell's research group
- Molecular neurodevelopment and neuro-oncology – Ola Hermanson group
- Research groups in anesthesiology and intensive care
- Gastrointestinal epidemiology – Jonas Ludvigsson's research group
- Health inequalities and minority stress – Richard Bränström's research group
- Research groups in microbiology
- Research groups in orthopaedics
- Hereditary hematological malignancies – Bianca Tesi team
- Lars Holmgren's Group
- Substance use prevention – Johanna Gripenberg's research group
- Perioperative care – Ulrica Nilsson's research group
- Mechanisms behind HIV-1 latency and rebound – Peter Svensson's group
- ENGAGE- Enacting health and change through everyday activity – Patomella group
- Diagnosis and treatment of children with asthma and allergy: from molecular diagnosis to intervention – Jon Konradsen's research group
- Translational research in childhood cancer and histiocytic diseases – Nikolas Herold Research group
- Reproductive Health/Reproductive Medicine – Kristina Gemzell Danielsson's research group
- Malin Holzmann's research group
- Radiation therapy/ Radiation Oncology – Pehr Lind Group
- Itziar Martinez Gonzalez group
- Reproductive endocrinology and metabolism – Elisabet Stener-Victorin's research group
- Tissue engineering – Magdalena Fossum team
- Inflammation and metabolism – Nicolas Pillon's team
- Neurobiology of Sensory Systems – François Lallemend group
- Experimental Traumatology Research Unit
- Genetic Modification of NK cells for Optimized Functions against Cancer – Arnika Wagner team
- NK cells in the development of adaptive immune responses – Benedict Chambers team
- Molecular pain research – Camilla Svensson's research group
- Brain Connectomics – Joana Pereira's research group
- Genomic Science and RNA Biology – Vicent Pelechano group
- Immunotherapy – Robert Harris' research group
- Infectious diseases/Venhälsan – Jaran Eriksen's research group
- Musculoskeletal disorders from a biopsychosocial perspective – Wim Grooten's research group
- Hjerling-Leffler group
- Respiratory and invasive infections and microbial pathogenesis – Birgitta Henriques-Normark Laboratory
- Multimodal Brain Imaging – Daniel Lundqvist's research group
- Obsessive - Compulsive and Related Disorders Across the Lifespan – David Mataix-Cols research group
- With a focus on proteins for better cancer treatments – Pär Nordlund's Group
- Precision cancer medicine and precision radiotherapy in lung cancer - from molecular mechanisms/biomarkers to clinical trials – Kristina Viktorsson's team
- Cancer Bioinformatics – Nick Tobin's Team
- Lipoproteins and the Immune System – Research group Stephen Malin
- Equity and Health Policy (EHP) – Ann Liljas' research group
- Effects of hypoxia in physiological and pathological contexts, Hypoxia Inducible Factors (HIF) – Randall Johnson's Group
- Chemistry II – Jesper Z. Haeggström group
- Genomic analysis of parasites and viruses, metagenomic sequencing – Björn Andersson's Group
- Translational control of cancer – Ola Larsson's Group
- Gastrointestinal Surgery KI SÖS – Gabriel Sandblom's research group
- Understanding the mechanisms behind hantavirus-mediated pathogenesis – Jonas Klingström group
- The Gynaecological research team – Elisabeth Epstein
- Understanding how life develops – Emma Andersson's Group
- Statistical methods in epidemiology – Paul Dickman's research group
- Neurons and Neural Networks – Ole Kiehn group
- Research groups in developmental biology
- Behavioral, Resilience and Digital Health in Pain – Rikard Wicksell’s research group
- Research groups in neurology
- Research groups in epidemiology, global public health and social medicine
- Nordic Brain Network – Miia Kivipelto's research group
- Resilience and mental health – Serhiy Dekhtyar group
- Forensic behavioral and neuroscience – Katarina Howner's Research Group
- Immune Engineering – Team Leo Hanke
- Chemical and physical exposure in the work environment and health – Jenny Selander's research group
- Mechanobiology of cardiac regeneration – Elif Eroglu's Group
- Tumors of the female genital organs – Miriam Mints' research group
- Psychoneuroimmunology – Mats Lekander's research group
- Mechanisms of malignant hematopoiesis – Pedro Moura team
- Fossati
- Neuroplasticity and Regeneration – Konstantinos Ampatzis group
- Paediatric cell and molecular biology – Hjalmar Brismar's research group
- Quantitative Biology of the Nucleus – Magda Bienko Group
- Cardiometabolic factors, brain aging, and dementia care – Weili Xu group
- Cognitive Neuroscience of Body and Self – Henrik Ehrsson Group
- Surgical Care Science – Pernilla Lagergren's research group
- Clinical and translational studies on viral hepatitis – Soo Aleman group
- Cell and Gene Therapy – Evren Alici group
- Personalized Medicine and Drug Development – Lauschke-lab
- Electrophysiological neuropharmacology – Göran Engberg's research group
- Mechanisms of Pain and Treatment – Eva Kosek's research group
- Functional (Epi)genomics of Neuroinflammatory Diseases – Maja Jagodic's research group
- Molecular basis of gene regulation of diseases – Carsten Daub's research group
- Health risk assessment methodology – Anna Beronius' group
- Medical genetics – Catharina Larsson's Group
- Translational Genetics of Neurodegenerative disease – Caroline Graff's research group
- Development, transcription factors, neurons, dopamine – Thomas Perlmann's Group
- Clinical and translational research within lupus and autoimmunity – Team Ioannis Parodis
- Cell cycle, mitotic entry, and DNA-damage checkpoints – Arne Lindqvist's Group
- Breast Surgery – Irma Fredriksson's research group
- Laura Orellana's Group
- Research group Ali Mirazimi
- Early drug discovery research in chronic inflammatory diseases – Research group Michael Sundström
- Integrative Physiology – Juleen Zierath's research group
- Simon Elsässer group
- Clinical Neuroimmunology and Immunomodulation – Fredrik Piehl's research group
- Understanding mechanisms of vaccination – Research group Karin Loré
- Systems regenerative neurobiology – Enric Llorens Group
- Clinicial Cancer Genomics – Johan Lindberg's research group
- Jeanette Hellgren Kotaleski group
- Brain control of food intake and body weight – Björn Meister group
- Research groups in endocrinology and diabetes
- Research groups in gastroenterology and hepatology
- Research groups in neurosciences
- Research groups in respiratory medicine and allergy
- Caring in Community Care – Zarina Nahar Kabir's research group
- Molecular mapping of the nervous system in health and disease – Jan Mulder group
- Child and adolescent psychiatry – Jens Högström's research group
- Genetic mechanisms of ageing – Maria Eriksson's research group
- Digital Psychiatry – Philip Lindner's research group
- Synaesthesia, autism and perception – Janina Neufeld's team
- Vascular complications in cardiometabolic disease – Team Zhichao Zhou
- Research groups in odontology
- Neuroimmunovascular biology – Harald Lund's team
- Dosenovic
Receptor biology and signaling – Schulte Lab
Our research aims to increase the understanding of basic pharmacology and underlying mechanisms of signal transduction and signaling specificity mediated by the Class Frizzled (FZD) receptors.
News
 Photo: Creative Commons CC0
Photo: Creative Commons CC0Make a donation to our research
Your support means a lot to the success of research. This allows us to go further in our efforts to improve human health through research and education.
About us
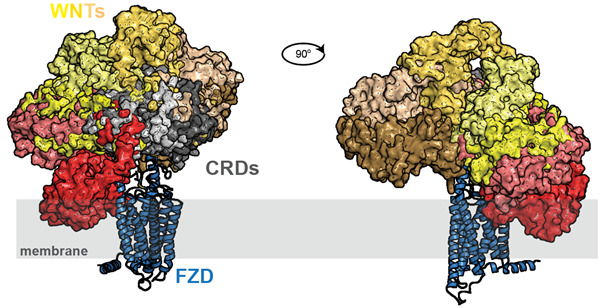
The class F of G protein-coupled receptors (GPCRs) consists of ten Frizzleds (FZD1-10) and Smoothened (SMO). The FZDs are - among other ligands - activated by 19 mammalian WNTs, a group of secreted lipoglycoproteins with an important role in embryonic development, stem cell regulation and human diseases, such as cancer. In the Schulte lab, we investigate how ligands activate FZDs in order to initiate intracellular changes. In this effort, we focus on ligand-receptor interaction, ligand-induced conformational changes in the receptor and aspects of conformational selection determining which downstream signaling pathways are activated. Thereby we hope to provide better insight into WNT-FZD binding mechanisms and selectivity and how intracellular signaling is initiated and specified by the individual FZD paralogues.
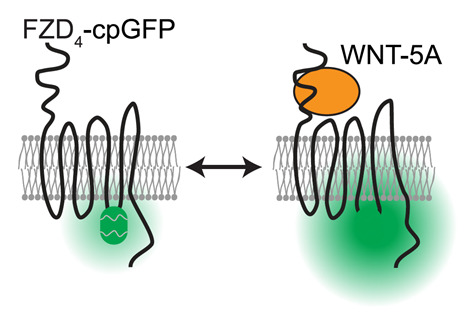
During the recent years, we have developed expertise in the use and design of genetically encoded biosensors that monitor ligand binding to FZDs, FZD conformational dynamics, FZD-effector binding and downstream effector activation. Furthermore, we combine these live cell assessments of the most upstream events of WNT-induced effects with in silico approaches such as molecular dynamics simulations and computational modelling to provide mechanistic insight into receptor activation and receptor complex dynamics on a molecular level.
One of the most important concepts that we have developed and that presents an important contribution to the field is the idea that FZDs are - similar to other GPCR – molecular machines that are defined by a structural flexibility and dynamics. Appreciation of this intrinsic flexibility is the basis for understanding receptor activation, receptor-mediated signal initiation and for targeting FZDs for therapy. Several important contributions from the Schulte lab have contributed to understand this central phenomenon (Wright and Kozielewicz et al. 2019; Kozielewicz et al. 2020; Turku et al. 2021; Kowalski-Jahn and Schihada et al. 2021; Schihada et al. 2021).
Our goal is to understand the complexity of WNT-induced events that determine the kinetics and nature of WNT signaling pathways governing embryonic development and tissue homeostasis. Since deregulated WNT signaling results in devastating diseases exemplified by many different and common forms of tumors (e g breast, intestinal, pancreatic cancer), bone disease, fibrosis and neurological disorders, we are convinced that targeting FZDs pharmacologically will open exciting strategies for the therapy of severe diseases.
Understanding how FZDs mediate WNT signaling to drive proliferation in a tumor or in a fibrotic tissue will allow us to design drugs that interfere with this message for therapeutic use. Our lab has shown for the first time that FZDs can be pharmacologically targeted by small molecule drugs (Kozielewicz et al. 2020) and we are currently developing this strategy to find drug-like compounds for anti-cancer treatment.
The computer simulation shows the interaction between FZD6 (white) and the small drug-like molecule SAG13 (purple).
The recent advances in our understanding of the details of FZD activation have been possible because of the development of biophysical assays that are suitable to dissect mechanistic aspects of signaling in an unprecedented manner. Furthermore, these assays are building the technological basis for drug screening efforts, which will explore the chemical matter on the quest for new drugs to treat cancer and other devastating human disorders.
Wright SC, Kozielewicz P, Kowalski-Jahn M, Petersen J, Bowin CF, Slodkowicz G, Marti-Solano M, Rodríguez D, Hot B, Okashah N, Strakova K, Valnohova J, Babu MM, Lambert NA, Carlsson J, Schulte G. A conserved molecular switch in Class F receptors regulates receptor activation and pathway selection. Nat Commun. 2019 Feb 8;10(1):667. doi: 10.1038/s41467-019-08630-2. PMID: 30737406
Kozielewicz P, Turku A, Bowin CF, Petersen J, Valnohova J, Cañizal MCA, Ono Y, Inoue A, Hoffmann C, Schulte G. Structural insight into small molecule action on Frizzleds. Nat Commun. 2020 Jan 21;11(1):414. doi: 10.1038/s41467-019-14149-3. PMID: 31964872
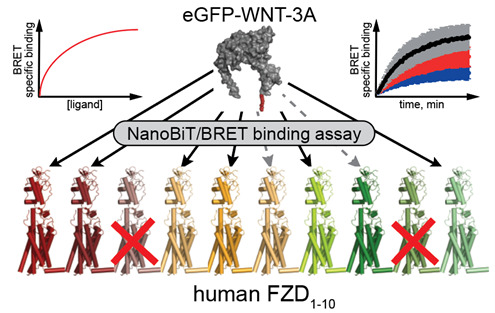
Wesslowski J, Kozielewicz P, Wang X, Cui H, Schihada H, Kranz D, Karuna M P, Levkin P, Gross JC, Boutros M, Schulte G, Davidson G. eGFP-tagged Wnt-3a enables functional analysis of Wnt trafficking and signaling and kinetic assessment of Wnt binding to full-length Frizzled. J Biol Chem. 2020 Jun 26;295(26):8759-8774. doi: 10.1074/jbc.RA120.012892. Epub 2020 May 7. PMID: 32381507
Schihada H, Kowalski-Jahn M, Turku A, Schulte G. Deconvolution of WNT-induced Frizzled conformational dynamics with fluorescent biosensors. Biosens Bioelectron. 2021 Apr 1;177:112948. doi: 10.1016/j.bios.2020.112948. Epub 2020 Dec 30. PMID: 33486136
Kozielewicz P, Shekhani R, Moser S, Bowin CF, Wesslowski J, Davidson G, Schulte G. Quantitative Profiling of WNT-3A Binding to All Human Frizzled Paralogues in HEK293 Cells by NanoBiT/BRET Assessments. ACS Pharmacol Transl Sci. 2021 May 11;4(3):1235-1245. doi: 10.1021/acsptsci.1c00084. eCollection 2021 Jun 11. PMID: 34151213
Turku A, Schihada H, Kozielewicz P, Bowin CF, Schulte G. Residue 6.43 defines receptor function in class F GPCRs. Nat Commun. 2021 Jun 24;12(1):3919. doi: 10.1038/s41467-021-24004-z. PMID: 34168128
Xu L, Chen B, Schihada H, Wright SC, Turku A, Wu Y, Han GW, Kowalski-Jahn M, Kozielewicz P, Bowin CF, Zhang X, Li C, Bouvier M, Schulte G, Xu F. Cryo-EM structure of constitutively active human Frizzled 7 in complex with heterotrimeric Gs. Cell Res. 2021 Jul 8. doi: 10.1038/s41422-021-00525-6. Online ahead of print. PMID: 34239071
Kowalski-Jahn M, Schihada H, Turku A, Huber T, Sakmar TP, Schulte G. Frizzled BRET sensors based on bioorthogonal labeling of unnatural amino acids reveal WNT-induced dynamics of the cysteine-rich domain. Sci Adv. 2021 Nov 12;7(46):eabj7917. doi: 10.1126/sciadv.abj7917. Epub 2021 Nov 10. PMID: 34757789
Funding
- Alex & Eva Wallström Stiftelse
- Cancerfonden
- Emil och Wera Cornells Stiftelse
- EUbOPEN
- Fernströms Stiftelsen
- FP7 Marie Skłodowska-Curie actions
- GACR (Czech Science Foundation)
- Hjärnfonden
- Jeanssons Stiftelse
- Karolinska Institutet
- KI/NIH Joint PhD
- KID
- Knut & Alice Wallenberg Stiftelse
- Max & Edith Follins Stiftelse
- Novonordiskfonden
- Signe & Olof Wallenius Stiftelse
- Signhild Engkvists Stiftelse
- Socialstyrelsen
- Stiftelsen Olle Engkvist Byggmästare
- STINT
- Tore Nilson Stiftelse
- Vetenskapsrådet
- Wenner-Gren Foundation
- Åhlén Stiftelse
- Åke Wiberg Stiftelse
Publications
Selected publications
- Article: JOURNAL OF MEDICINAL CHEMISTRY. 2024;67(24):22332-22341Kinsolving J; Gratz L; Voss JH; Loew B; Shorter E; Jude B; Lanner JT; Loeber S; Gmeiner P; Schulte G
- Article: BRITISH JOURNAL OF PHARMACOLOGY. 2024;181(20):3819-3835Gratz L; Sajkowska-Kozielewicz JJ; Wesslowski J; Kinsolving J; Bridge LJ; Petzold K; Davidson G; Schulte G; Kozielewicz P
- Article: HEPATOLOGY. 2024;79(6):1337-1351Oliva-Vilarnau N; Beusch CM; Sabatier P; Sakaraki E; Tjaden A; Graetz L; Buettner FA; Dorotea D; Nguyen M; Bergqvist F; Sundstrom Y; Mueller S; Zubarev RA; Schulte G; Tredup C; Gramignoli R; Tietge UJF; Lauschke VM
- Review: TRENDS IN PHARMACOLOGICAL SCIENCES. 2024;45(5):419-429Schulte G; Scharf MM; Bous J; Voss JH; Gratz L; Kozielewicz P
- Article: NATURE COMMUNICATIONS. 2023;14(1):4573Gratz L; Kowalski-Jahn M; Scharf MM; Kozielewicz P; Jahn M; Bous J; Lambert NA; Gloriam DE; Schulte G
- Article: SCIENCE SIGNALING. 2023;16(779):eabo4974Bowin C-F; Kozielewicz P; Gratz L; Kowalski-Jahn M; Schihada H; Schulte G
- Letter: CELL RESEARCH. 2021;31(12):1311-1314Xu L; Chen B; Schihada H; Wright SC; Turku A; Wu Y; Han G-W; Kowalski-Jahn M; Kozielewicz P; Bowin C-F; Zhang X; Li C; Bouvier M; Schulte G; Xu F
- Article: SCIENCE ADVANCES. 2021;7(46):eabj7917Kowalski-Jahn M; Schihada H; Turku A; Huber T; Sakmar TP; Schulte G
- Article: NATURE COMMUNICATIONS. 2021;12(1):3919Turku A; Schihada H; Kozielewicz P; Bowin C-F; Schulte G
- Article: ACS PHARMACOLOGY AND TRANSLATIONAL SCIENCE. 2021;4(3):1235-1245Kozielewicz P; Shekhani R; Moser S; Bowin C-F; Wesslowski J; Davidson G; Schulte G
- Article: BIOSENSORS & BIOELECTRONICS. 2021;177:112948Schihada H; Kowalski-Jahn M; Turku A; Schulte G
- Article: JOURNAL OF BIOLOGICAL CHEMISTRY. 2020;295(26):8759-8774Wesslowski J; Kozielewicz P; Wang X; Cui H; Schihada H; Kranz D; Karuna PM; Levkin P; Gross JC; Boutros M; Schulte G; Davidson G
- Article: NATURE COMMUNICATIONS. 2020;11(1):414Kozielewicz P; Turku A; Bowin C-F; Petersen J; Valnohova J; Canizal MCA; Ono Y; Inoue A; Hoffmann C; Schulte G
- Article: MOLECULAR PHARMACOLOGY. 2020;97(1):23-34Kozielewicz P; Bowin C-F; Turku A; Schulte G
- Article: JOURNAL OF BIOLOGICAL CHEMISTRY. 2019;294(31):11677-11684Bowin C-F; Inoue A; Schulte G
- Article: NATURE COMMUNICATIONS. 2019;10(1):667Wright SC; Kozielewicz P; Kowalski-Jahn M; Petersen J; Bowin C-F; Slodkowicz G; Marti-Solano M; Rodriguez D; Hot B; Okashah N; Strakova K; Valnohova J; Babu MM; Lambert NA; Carlsson J; Schulte G
- Article: SCIENCE SIGNALING. 2018;11(559):eaar5536Wright SC; Canizal MCA; Benkel T; Simon K; Le Gouill C; Matricon P; Namkung Y; Lukasheva V; Koenig GM; Laporte SA; Carlsson J; Kostenis E; Bouvier M; Schulte G; Hoffmann C
- Article: JOURNAL OF BIOLOGICAL CHEMISTRY. 2018;293(48):18477-18493Strakova K; Kowalski-Jahn M; Gybel T; Valnohova J; Dhople VM; Harnos J; Bernatik O; Ganji RS; Zdrahal Z; Mulder J; Lindskog C; Bryja V; Schulte G
- Article: JOURNAL OF BIOLOGICAL CHEMISTRY. 2018;293(46):17875-17887Valnohova J; Kowalski-Jahn M; Sunahara RK; Schulte G
- Article: CELLULAR SIGNALLING. 2017;38:85-96Strakova K; Matricon P; Yokota C; Arthofer E; Bernatik O; Rodriguez D; Arenas E; Carlsson J; Bryja V; Schulte G
- Article: NATURE COMMUNICATIONS. 2017;8(1):226Petersen J; Wright SC; Rodriguez D; Matricon P; Lahav N; Vromen A; Friedler A; Stromqvist J; Wennmalm S; Carlsson J; Schulte G
- Article: CELLULAR SIGNALLING. 2017;32:93-103Hot B; Valnohova J; Arthofer E; Simon K; Shin J; Uhlen M; Kostenis E; Mulder J; Schulte G
- Article: MOLECULAR PHARMACOLOGY. 2016;90(4):447-459Arthofer E; Hot B; Petersen J; Strakova K; Jager S; Grundmann M; Kostenis E; Gutkind JS; Schulte G
- Article: EXPERIMENTAL CELL RESEARCH. 2015;339(2):280-288Dijksterhuis JP; Arthofer E; Marinescu VD; Nelander S; Uhlen M; Ponten F; Mulder J; Schulte G
- Article: JOURNAL OF BIOLOGICAL CHEMISTRY. 2015;290(11):6789-6798Dijksterhuis JP; Baljinnyam B; Stanger K; Sercan HO; Ji Y; Andres O; Rubin JS; Hannoush RN; Schulte G
- Article: CELLULAR SIGNALLING. 2014;26(9):1943-1949Kilander MBC; Dahlstrom J; Schulte G
- Article: FASEB JOURNAL. 2014;28(5):2293-2305Kilander MBC; Petersen J; Andressen KW; Ganji RS; Levy FO; Schuster J; Dahl N; Bryja V; Schulte G
- Article: JOURNAL OF NEUROCHEMISTRY. 2013;125(6):803-808Halleskog C; Schulte G
- Article: CELLULAR SIGNALLING. 2013;25(4):822-828Halleskog C; Schulte G
- Article: JOURNAL OF NEUROINFLAMMATION. 2012;9:111Halleskog C; Dijksterhuis JP; Kilander MBC; Becerril-Ortega J; Villaescusa JC; Lindgren E; Arenas E; Schulte G
- Article: ACTA PHYSIOLOGICA. 2011;203(3):363-372Kilander MBC; Halleskog C; Schulte G
- Article: CELLULAR SIGNALLING. 2011;23(3):550-554Kilander MBC; Dijksterhuis JP; Ganji RS; Bryja V; Schulte G
- Article: GLIA. 2011;59(1):119-131Halleskog C; Mulder J; Dahlstrom J; Mackie K; Hortobagyi T; Tanila H; Puli LK; Faerber K; Harkany T; Schulte G
- Article: EMBO REPORTS. 2008;9(12):1244-1250Bryja V; Schambony A; Cajanek L; Dominguez I; Arenas E; Schulte G
- Published conference paper: ACTA PHYSIOLOGICA. 2007;190(1):55-61Bryja V; Cajanek L; Grahn A; Schulte G
- Article: PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 2007;104(16):6690-6695Bryja V; Gradl D; Schambony A; Arenas E; Schulte G
- Show more
Review articles
Molecular Pharmacology of Class F Receptor Activation.
Kozielewicz P, Turku A, Schulte G
Mol Pharmacol 2020 02;97(2):62-71
Structural insight into Class F receptors - What have we learnt regarding agonist-induced activation?
Schulte G, Kozielewicz P
Basic Clin. Pharmacol. Toxicol. 2019 Mar
Frizzleds as GPCRs - More Conventional Than We Thought!
Schulte G, Wright SC
Trends Pharmacol. Sci. 2018 09;39(9):828-842
Frizzleds and WNT/β-catenin signaling--The black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics.
Schulte G
Eur. J. Pharmacol. 2015 Sep;763(Pt B):191-5
WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3.
Dijksterhuis JP, Petersen J, Schulte G
Br. J. Pharmacol. 2014 Mar;171(5):1195-209
International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors.
Schulte G
Pharmacol. Rev. 2010 Dec;62(4):632-67
beta-Arrestins - scaffolds and signalling elements essential for WNT/Frizzled signalling pathways?
Schulte G, Schambony A, Bryja V
Br. J. Pharmacol. 2010 Mar;159(5):1051-8
The Frizzled family of unconventional G-protein-coupled receptors.
Schulte G, Bryja V
Trends Pharmacol. Sci. 2007 Oct;28(10):518-25
Podcasts
The Dr. GPCR podcast #46: Gunnar Schulte interviewed by Dr Yamina Berchiche.
Full publication list
Staff and contact
Group leader
- Gunnar SchulteProfessor | Docent
All members of the group
- Julien BousAffiliated to Research
- Farouk GhanemResearch Assistant
- Laura JahrstorferAffiliated to Research
- Julia KinsolvingPhd Student
- Paweł KozielewiczAssistant Professor | Team Leader | Docent
- Philipp KölblAffiliated to Research
- Gaia MartinianiAffiliated to Research
- Magdalena ScharfPostdoctoral Researcher
- Nini SchotmanAffiliated to Research
- Gunnar SchulteProfessor | Docent
- Rawan ShekhaniAffiliated to Research
- Rhiannon SkyeAffiliated to Research
Alumni
- Maria Kowalski-Jahn, postdoc
- Ainoleena Turku, postdoc
- Shane Wright, postdoc
- Elisa Arthofer, PhD
- Fredrik Brand, Master's student from the Lausitz University of Applied Sciences, Germany
- Jenny Dahlström, Med kand, master's student
- Jacomijn Dijksterhuis, PhD
- Carina Halleskog, PhD
- Rober Helbig, PhD, postdoctoral fellow
- Belma Hot, PhD
- Michaela Kilander, PhD
- Natália Assaife Lopes, PhD student, Faculty of Medicine, University of Lisbon, Portugal
- Tobias Ludwig, Student from the Free University of Berlin, Germany
- Javier Becerril Ortega, PhD, postdoctoral fellow
- Julian Petersen, PhD
- Tilman Polonio, Student from Heidelberg University, Germany
- Jana Valnohova, PhD
- Alice Weithäuser, Master's student from the Free University of Berlin, Germany
- Carl Fredrik Bowin (PhD student)
- Hannes Schihada, postdoc
Team - Molecular pharmacology of GPCRs
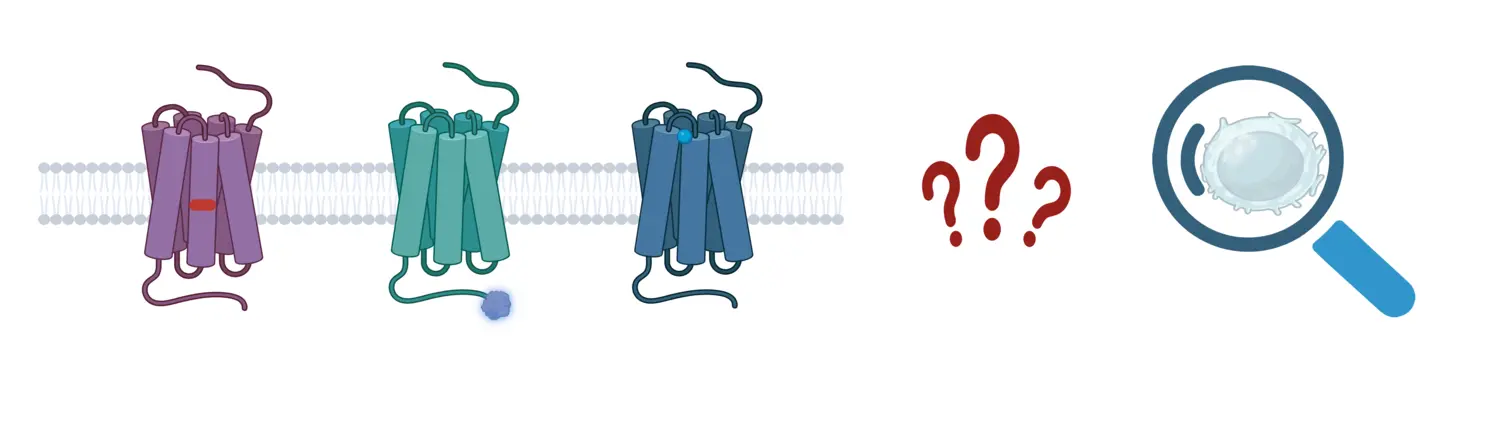
We are interested in understanding the role of GPCRs in health and disease, with a focus on cancer and obesity. Of particular interest to us are understudied orphan GPCRs. These receptors bear untapped therapeutic potential and a lof of them are highly expressed or mutated in these diseases. To shed light on the functions of orphan GPCRs in these conditions, we aim to employ an integrative approach. We will study these proteins in different systems, ranging from HEK293 cells to patients' samples, and investigate phenomena at different levels: from receptor conformational changes to impact on cell phenotype.
Our methodologies include but are not limited to:
- Bioluminescence resonance energy transfer (BRET)
- CRISPR-Cas9 genome editing
- Molecular modelling with ligand docking and molecular dynamics simulations
- Proximal labelling techniques
- Analysis of large genomic datasets
Projects:
- Analysis of the role of orphan GPCRs in cancer
- Investigations into orphan GPCRs as regulators of metabolism
- Understandng the pharmacology of Smoothened
Team leader
Paweł Kozielewicz
Assistant ProfessorFarouk Ghanem
Research AssistantRhiannon Skye
Affiliated to ResearchLaura Jahrstorfer
Affiliated to ResearchAlumni
Hellen Röttgen
Alexander De Rosa
Alesia Tsalikou
Choi Har Tsang
Louise Andersson
Salina Chhetri
Holly Brittain
Conference contribution
2023
Gunnar Schulte is an invited speaker at the 11th MPGPCR (Molecular Pharmacology of GPCRs) meeting in Melbourne held the 15th-17th of Nov 2023. https://www.monash.edu/pharm/about/events/mpgpcr-2023
Gunnar Schulte contributes to the Nordic Symposium at the 19th World Congress of Basic & Clinical Pharmacology 2023 (WCP2023) with a talk on the 5th of July 2023.
https://wcp2023.org/
The Schulte lab will be well-represented at the Gordon Research Conference (GRC) and Gordon Research Seminar (GRS)in Molecular Pharmacology in June 11-16 2023. Gunnar Schulte and Lukas Graetz are invited speakers.
https://www.grc.org/molecular-pharmacology-conference/2023/
https://www.grc.org/molecular-pharmacology-grs-conference/2023/
2022
4GPCRnet – International Symposium Sept 26-29, 2022 (Leipzig, Germany) – Gunnar Schulte is invited speaker
https://research.uni-leipzig.de/sfb1423/4gpcrnet2022/
25th Jubilee Meeting on Signal Transduction Nov 2 - 4, 2022 (Weimar, Germany) – Gunnar Schulte is invited speaker (Hot Topics in Signal Transduction)
https://sigtrans.de/meeting-2022
2019
G protein-coupled receptors - from physiology to drugs
8th International meeting.
9-11 October 2019, Montpellier, France.
EB2019 New Opportunities in Targeting WNT Signaling
The ASPET Annual Meeting is where pharmacology meets experimental biology in ONE community.
8 April 2019, Orlando, Florida, USA.
Pharmacology 2019
Organised by The Swedish Society for Pharmacology, Clinical Pharmacology and Therapeutics, the Swedish Society of Medicine.
2-3 April 2019 at Karolinska Institutet, Sweden.
The 2019 Gordon Research Conference on Molecular Pharmacology will highlight the latest advances in understanding G protein-coupled receptors (GPCRs) and how they mediate regulation of physiological processes. GPCRs are well established effective therapeutic targets and presentations will focus on how we can further improve therapeutic development across diverse disease states, including cancer, metabolic disorders, cardiovascular disease, and pain.
10-15 February 2019, Ventura Beach Marriott, Ventura, CA, US
2018
GPCR Pharmacology: The Next Generation
The Royal Danish Academy of Sciences and Letters
31 October - 2 November 2018, Copenhagen, Denmark
2017
Workshop organised by ConfometRX Research Foundation and Monash Institute of Pharmaceutical Sciences, Melbourne, Australia. Key themes for the 2017 Workshop were advances in structural understanding of GPCRs, novel signaling paradigms, the intersection of computational advances and chemical biology, biologics, and preclinical and clinical translation.
5-9 December 2017, Sheraton Kona, Hawaï
2016
6th BPS Focused Meeting on Cell Signalling
Organised by the British Pharmacological Society.
18-19 April 2016, University of Leicester, Stamford Court, United Kingdom
2015
GRS Molecular Pharmacology (Gordon Research Seminar, GRS) - "New Fronties in GPCR Signaling: From Biased Agonism to Disease Progression"
The Gordon Research Seminar on Molecular Pharmacology is a unique forum for graduate students, post-docs, and other scientists with comparable levels of experience and education to present and exchange new data and cutting edge ideas.
31 January - 1 February, 2015, Ventura Beach Marriott, USA
Master's and PhD projects

2021
Doctoral thesis Carl- Fredrik Bowin (2021-11-12)
“WNT/Frizzled signaling – Illuminating the road towards pathway selectivity”

2018
Doctoral thesis Jana Valnohova (2018-11-09)

2017
Doctoral thesis Belma Hot (2017-04-28)
"Molecular aspects of WNT/Frizzled signalling in brain angiogenesis"

Doctoral thesis Elisa Arthofer (2017-03-24)

Doctoral thesis Katerina Straková
"Interaction of Class Frizzled receptors with G proteins and Dishevelled"

2016
Doctoral thesis Julian Petersen (2016-01-29)
"Structural and functional analysis of WNT receptors : with emphasis on FZD6"
2015
Doctoral thesis Jacomijn Dijksterhuis (2015-03-06)
"WNT/FZD signaling : an odyssey from molecular pharmacology to brain (patho)physiology"

2013
Doctoral thesis Michaela Kilander (2013-09-06)
Doctoral thesis Carina Halleskog (2013-06-14)
"WNT signaling in microglia : WNTs as novel regulators of microglia"

2007
Doctoral thesis Frand Brand (2007-10-31)
"Agonist-induced Signaling and Endocytosis of the Adenosine A2A Receptor"

2002
Doctoral thesis Gunnar Schulte (2002-11-08)
"Adenosine receptor signaling and the activation of mitogen-activated protein kinases"
Vacant positions
I would be happy to assist with applications for external support in form of postdoctoral or PhD student fellowships.
I strongly encourage fellows eligible for postdoctoral application to Hjärnfonden and SSMF to contact me with a letter of interest, CV and letters of recommendation.