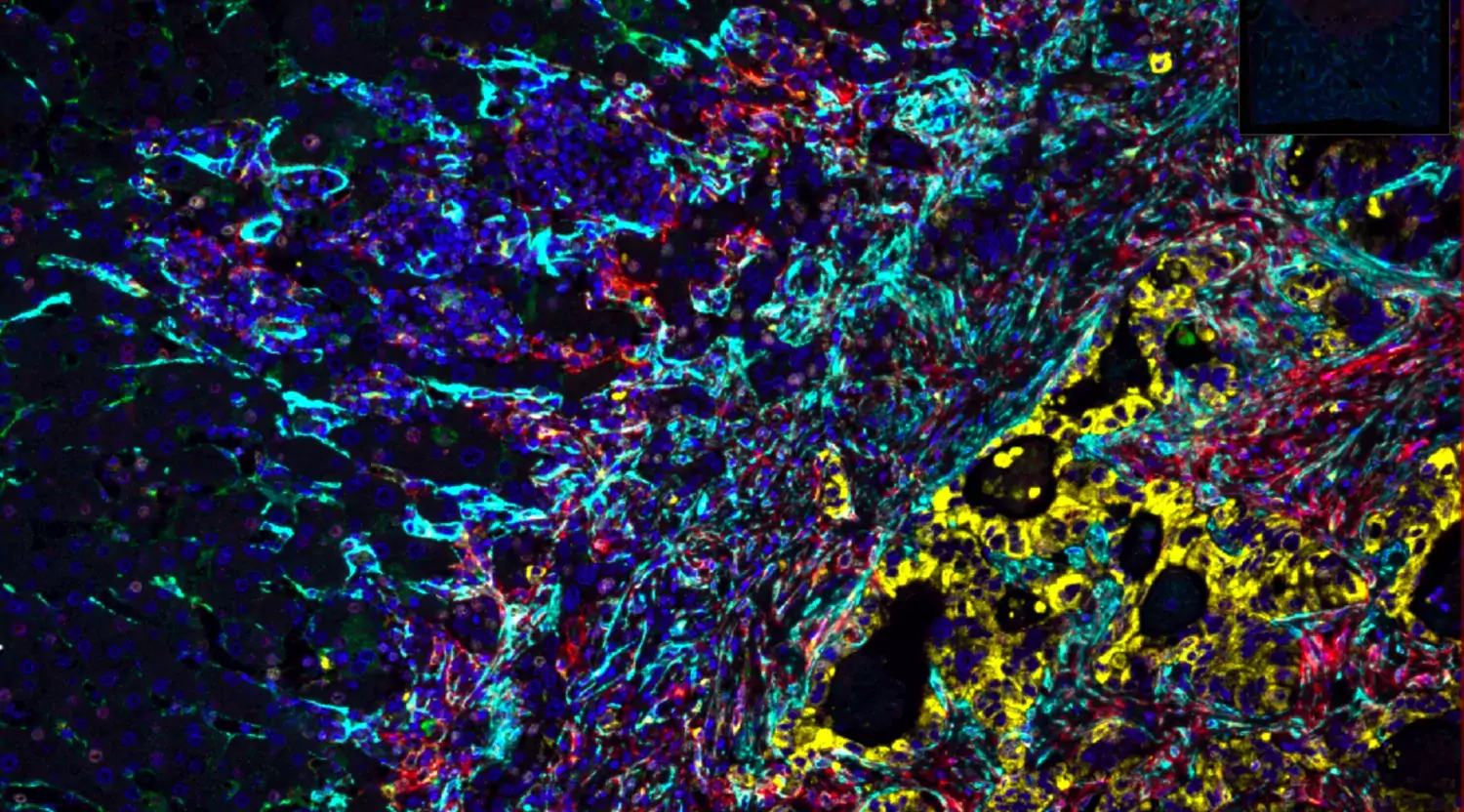
Colorectal and Pancreatic Cancer
Colorectal and pancreatic cancers account for more than 5000 new cancer cases every year in Sweden alone.
The aggressiveness of both tumors is tightly controlled by their microenvironment, such that direct cell-cell interactions and stromal signaling processes regulate differentiation and proliferation of the tumor cells.
We use genetic mouse models, ex vivo cell culture with live cell 2-photon imaging, and single cell RNA sequencing to chart and to experimentally modify cell-cell interactions in these cancer types. We are building large clinical cohorts of liver metastases patients and pancreatic cancer patients at Karolinska University Hospital to validate our findings.
Our goal is to bridge clinical and basic research in order to uncover ways of exploiting cellular interactions for cancer therapy.
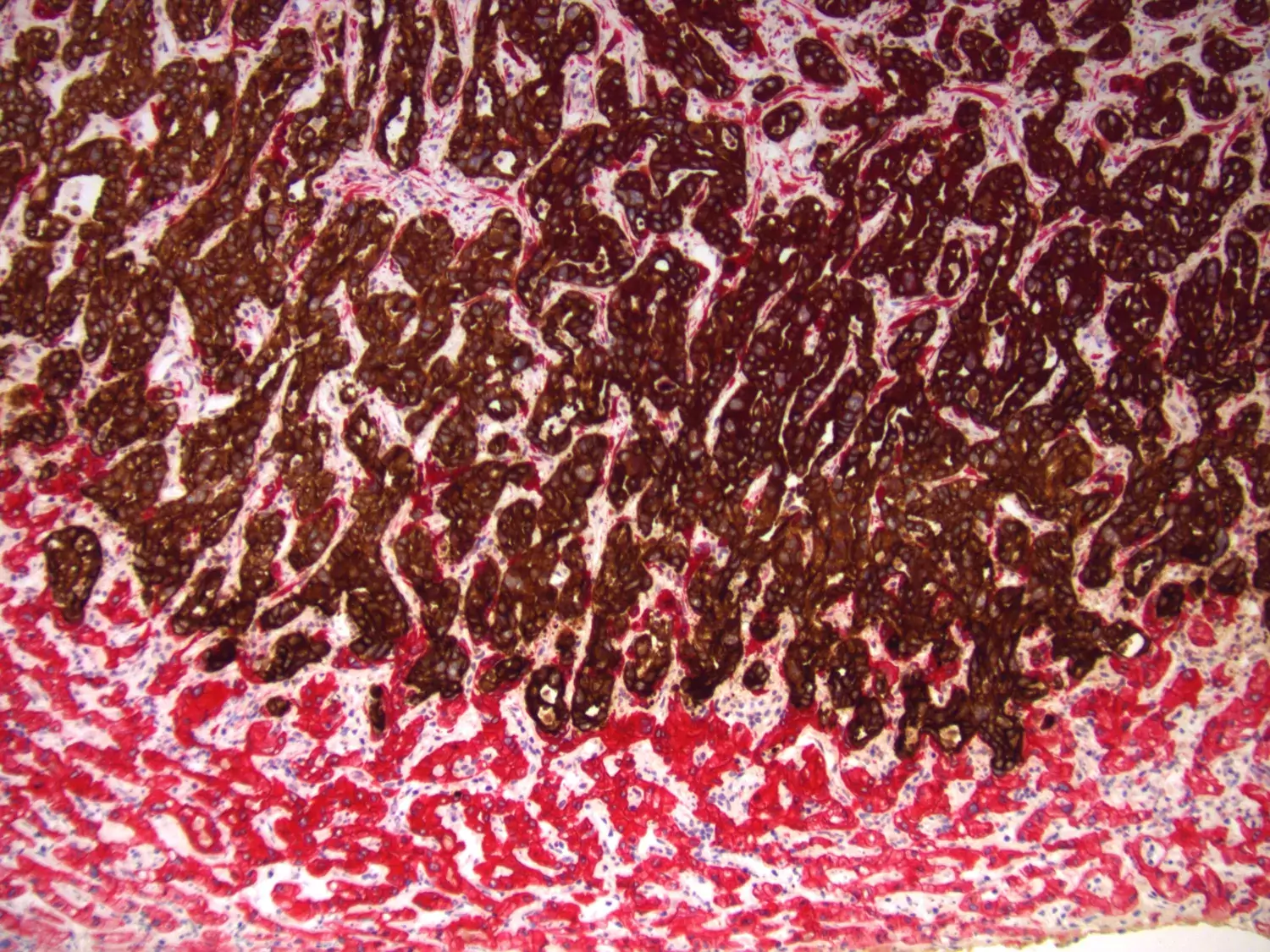
Liver Metastases
When tumor cells metastasise to distant organs, they encounter entirely new interaction partners.
Most metastases of gastrointestinal cancers are found in the liver, where tumor cells meet novel cell types, such as hepatocytes, hepatic stellate cells, liver sinusoid cells, and Kupffer cells.
Little is known about tumor-microenvironment interactions in metastases, despite their clinical importance. Therefore, we have developed models to alter the metastatic microenvironment in the liver, and we analyse clinical samples from patients with liver metastases. Our aim is to understand how cancer cells progress in the liver parenchyma, in order to find ways to slow down or even revert this process.