Research
Effect of intestinal parasites on distal immune responses
Parasitic infections are still a big problem in many parts of the world, negatively affecting the health of the poorest and most vulnerable populations. On the other hand, changes in infectious exposure and microbiota over the past century, most evidently in the Western world the loss of instestinal parasites, coincide with a steady increase in immune mediated diseases. Since parasitic infections normally have co-evolved with their host and are often chronic in their nature, their presence is and has been a driving force in shaping the host immune system both at individual and population level.
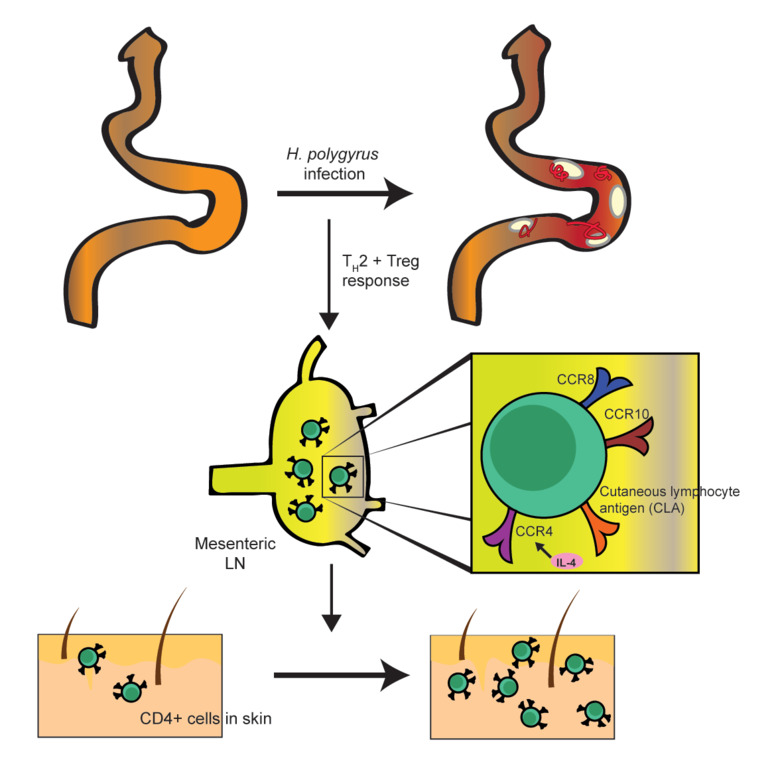
Understanding the marks microorganisms leave in the host is highly relevant to understanding differentiation of T cells in tissues and the implication this has on the ability to respond to subsequent infections and vaccination or contributing to the over-reactivity seen in autoimmune diseases. It has been demonstrated that intestinal worm infection can alter immune responses to other pathogens, vaccines, autoantigens, allergens and contact sensitizers. These effects have been believed to be due to expansion of TREG and/or TH2 cells, changes in the intestinal microbiota or its metabolome, or, as we have shown, atrophy of superficial lymph nodes (Feng 2018). Yet, the full spectrum of helminth-induced effects on the immune system remains to be elucidated.
Research in my group address how parasitic infection affect formation and maintainance of immunity to co-infections (e.g. Mycobacteria, Leishmania, Influenza virus) and vaccines. We have focused on immune responses distal to the site of infection and the ability of intestinal worms to modulate barrier tissue such as the skin. We use the intestinal nematode Heligmosomoides polygurus as model to address the impact of gut infection on T cells seeding into tissues and the longlasting effects these cells have on their local environment. Specifically, we aim to defined factors that determine T cell distribution and colonization of tissue following infection and the implication this may have on tissue immunity.
Understanding factors that influence the durability of vaccine-induced immunity and how this may differ in populations with different infectious burden is critical for making informed decisions on vaccination and boosting strategies relevant to both human and animal disease and welfare.
Development of antimicrobial resistance in parasites
Epigenetic modifications can allow fast adaptation to environmental changes (such as drugs) and may precede the mutations in development of antimicrobial resistance. This may be particularly relevant for larger parasites, like worms. In collaboration with KI colleagues Björn Andersson, Ulf Ribacke and Mats Wahlgren, as well as two colleagues from Uppsala University Andrea Hinas and Staffan Svärd, we address drug development and epigenetic modifications in several eukaryotic parasites including worms.
Visceral Leishmaniasis
In collaboration with Banaras Hindu University, I am involved in studies of Visceral leishmaniasis in Bihar state, India, in a project aiming to understand immune responses, in particular T cells responses that determine outcome and control of Leishmania donovani parasites.
For more information
Selected publications
Intestinal helminth infection transforms the CD4+ T cell composition of the skin.
Classon CH, Li M, Clavero AL, Ma J, Feng X, Tibbitt CA, Stark JM, Cardoso R, Ringqvist E, Boon L, Villablanca EJ, Rothfuchs AG, Eidsmo L, Coquet JM, Nylén S
Mucosal Immunol 2021 Dec;():
Single-Cell RNA Sequencing of the T Helper Cell Response to House Dust Mites Defines a Distinct Gene Expression Signature in Airway Th2 Cells.
Tibbitt CA, Stark JM, Martens L, Ma J, Mold JE, Deswarte K, Oliynyk G, Feng X, Lambrecht BN, De Bleser P, Nylén S, Hammad H, Arsenian Henriksson M, Saeys Y, Coquet JM
Immunity 2019 07;51(1):169-184.e5
Intestinal nematode infection exacerbates experimental visceral leishmaniasis.
Classon C, Feng X, Eidsmo L, Nylén S
Parasite Immunol 2019 04;41(4):e12618
Resident T Cells in Resolved Psoriasis Steer Tissue Responses that Stratify Clinical Outcome.
Gallais Sérézal I, Classon C, Cheuk S, Barrientos-Somarribas M, Wadman E, Martini E, Chang D, Xu Landén N, Ehrström M, Nylén S, Eidsmo L
J Invest Dermatol 2018 08;138(8):1754-1763
Atrophy of skin-draining lymph nodes predisposes for impaired immune responses to secondary infection in mice with chronic intestinal nematode infection.
Feng X, Classon C, Terán G, Yang Y, Li L, Chan S, Ribacke U, Rothfuchs AG, Coquet JM, Nylén S
PLoS Pathog 2018 05;14(5):e1007008
PPAR-γ promotes type 2 immune responses in allergy and nematode infection.
Chen T, Tibbitt CA, Feng X, Stark JM, Rohrbeck L, Rausch L, Sedimbi SK, Karlsson MCI, Lambrecht BN, Karlsson Hedestam GB, Hendriks RW, Chambers BJ, Nylén S, Coquet JM
Sci Immunol 2017 Mar;2(9):
Chronic Gastrointestinal Nematode Infection Mutes Immune Responses to Mycobacterial Infection Distal to the Gut.
Obieglo K, Feng X, Bollampalli VP, Dellacasa-Lindberg I, Classon C, Österblad M, Helmby H, Hewitson JP, Maizels RM, Gigliotti Rothfuchs A, Nylén S
J Immunol 2016 Mar;196(5):2262-71
BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAMlow CD11bhigh Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming.
Bollampalli VP, Harumi Yamashiro L, Feng X, Bierschenk D, Gao Y, Blom H, Henriques-Normark B, Nylén S, Rothfuchs AG
PLoS Pathog 2015 Oct;11(10):e1005206
Leishmania specific CD4 T cells release IFNγ that limits parasite replication in patients with visceral leishmaniasis.
Kumar R, Singh N, Gautam S, Singh OP, Gidwani K, Rai M, Sacks D, Sundar S, Nylén S
PLoS Negl Trop Dis 2014 Oct;8(10):e3198
CD8 T cell exhaustion in human visceral leishmaniasis.
Gautam S, Kumar R, Singh N, Singh AK, Rai M, Sacks D, Sundar S, Nylén S
J Infect Dis 2014 Jan;209(2):290-9
