Our Research
Immune responses determining the outcome of infection with Mycobacterium tuberculosis
Tuberculosis (TB), caused by infection with Mycobacterium tuberculosis (Mtb), remains a leading public health problem. While most individuals infected with Mtb remain asymptomatic, in 2020, ten million individuals fell ill with this disease and 1.5 million died1.
It is not completely understood why some individuals will develop a life-threatening disease while others harbour a lifelong asymptomatic infection, but the crosstalk of mycobacteria and cellular immune components plays a major role. HIV and Diabetes Mellitus (DM) are risk factors for development of diseases. DM has been associated with TB for centuries, however, in the complex relation between TB and DM and their respective treatments, many important topics have been poorly studied or not studied at all.
Infection with Mtb occurs when the inhaled bacilli are phagocytized by resident lung alveolar macrophages (1). Infected cells recruit mononuclear phagocytes to the infection site, forming a nascent granuloma. During the subclinical stage of infection, the granuloma provides the immune environment required for the containment of bacteria. Although innate immune responses are initialized, Mtb-specific T cells are crucial for the granuloma maturation, maintenance and control of the bacterial spread (2). However, if due to impaired immunity the integrity of the granuloma is lost, reactivation of Mtb leads to the destruction of the lung structure and to the transmission of Mtb to other humans. Thus, the TB granuloma is the niche in which bacilli either can grow and disseminate or in which host cells interact to prevent bacterial dissemination. In the granuloma, macrophage activation by antigen-specific T cells is crucial for a successful control of the bacterial spread.
AIMS
We explore immune mechanisms of bacterial control and to topologically define immune-bacterial interactions in the lung in experimental or clinical Tuberculosis. T-cell immunity is critical for control of the infection with M. tuberculosis (Mtb).
We propose to study the spatial coordination of T cells with other cell types and the balance of activating and inhibitory immune responses in situ in association to the morphology of the lesion.
Using spatially resolved transcriptomics technology we will simultaneously target 256 immune and mycobacterial transcripts in pulmonary sections from patients with Tuberculosis (TB), TB and Diabetes, and sarcoidosis, and thereby define the distinct topography of the granuloma in these conditions. We have successfully applied in situ sequencing to delineate an immunological landscapes of the granuloma in lungs from mice at different time points after infection; in lungs developing encapsulated necrotic or non-encapsulated cellular granulomas; in different granulomas of the same lung and in different areas within the lesions.
We aim to understand the role played by major molecular pathways regulate the outcome of infection Mycobacterium tuberculosis in experimental models by controlling the protective activity of T cells and macrophages.
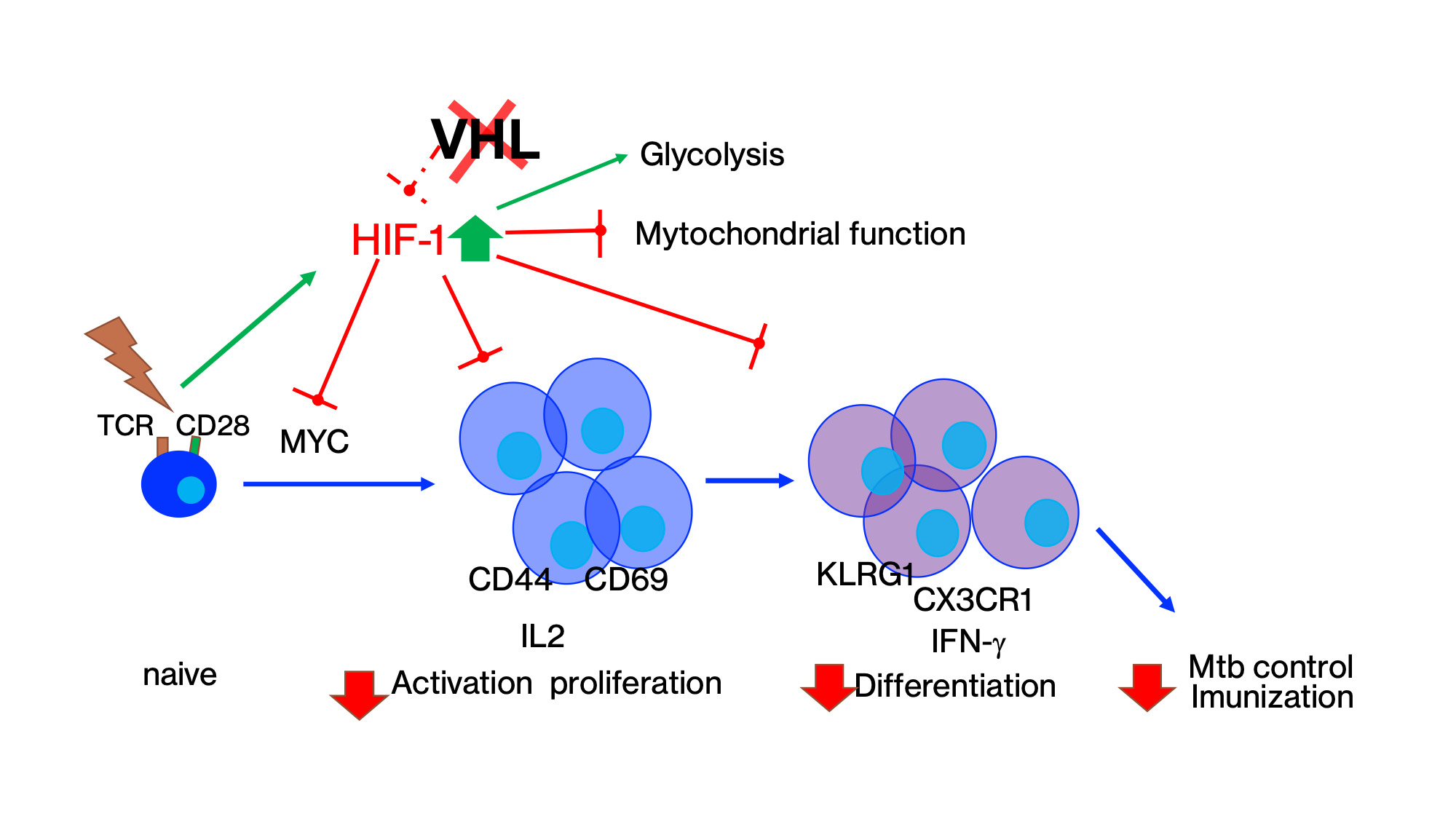
The role of STAT3 and HIF-1α in T cells during Mtb infection of otherwise healthy or diabetic mice are investigated using genetically manipulated animals.
The hypoxia-inducible factors (HIF) are central regulators of the cellular responses to hypoxia. We show that HIF-1 stabilization in T cells impairs the activation, cell cycle progression, proliferation, and differentiation of CD4 T cells, and the control of infection with M. tuberculosis. Liu et al submitted.
Full publication list
 Photo: Fredrik Persson
Photo: Fredrik PerssonAnnelie Brauner project
The host – microbe interaction is of prime importance to understand why some people get recurrent or complicated infections, while others do not. Our primary focus is infections in the urinary tract, and the skin. We specifically investigate the early defence mechanisms which protect the body from invading bacteria, and how we can exploit these natural strategies for possible therapeutic purposes.
 Photo: Saknas
Photo: SaknasUte Römling Group
Ute Römling Group researches in Multicellular behavior in Enterobacteriaceae, Cyclic di-GMP signaling, Pseudomonas aeruginosa clone C – a world wide prevalent clone and Candida parapsilosis.
Support our research
 Photo: Chokniti Khongchum
Photo: Chokniti KhongchumMake a donation to our research at MTC
Your support means a lot to our success. This allows us to go further in our efforts to improve human health through research and education.
Read here how you can make a donation via Swish.

