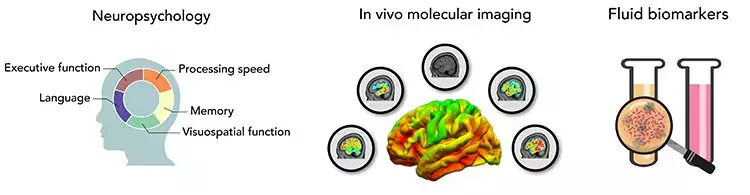
Proteinopathies are common features of neurodegenerative brain disorders and used as an umbrella term for different brain disorders, commonly leading to dementia. The proteinopathies are characterized by the accumulation or change in function of different specific proteins within or outside the neurons in the brain parenchyma, and presenting with different regional distributions in the brain.
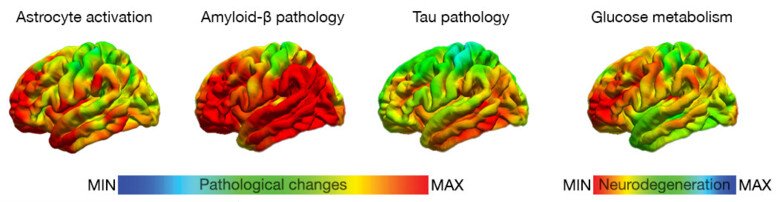
Alzheimer’s disease (AD), the leading cause of dementia, is characterized by the deposition of amyloid-β (Aβ) plaques and hyperphosphorylated tau protein tangles. In addition, multiple factors including the activation of astrocytes and microglia and neurovascular processes play interactive roles leading to synaptic dysfunction, brain atrophy and cognitive impairment. Many other neurodegenerative diseases, non-AD tauopathies, are pathologically characterized by the deposition of tau tangles, as well as complex factors involving other protein deposits.
AD has a long presymptomatic period during which neuropathology starts to accumulate decades before clinical onset and these processes can be quantifiable in subjects using brain imaging techniques such as positron emission tomography (PET) and magnetic resonance imaging (MRI) which can be used as early biomarkers
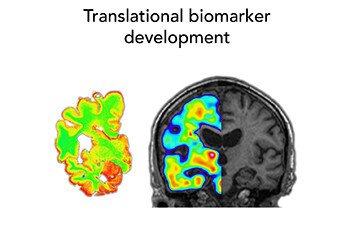
Our research group has a translational approach using in vitro and in vivo molecular brain imaging techniques, and our aim is to characterize the complex pathophysiological features of neurodegenerative diseases, with a strong focus on AD but also other proteinopathies, in order to develop new early diagnostic markers and new drug targets for early intervention in disease process.
Collaborations
We have extensive collaboration within our own department (NVS), with other departments at KI as well as other research groups at other universities in Sweden including Uppsala, Gothenburg and Stockholm. We also have strong collaboration with several foreign universities including University of Helsinki, University of Turku, University of Manchester, Indiana University School of Medicine, Denver University, Geneva university, Harvard Medical School, La Pitie Salpetriere-Charles Foix hospital, Amsterdam University, McGill University, and Universitat Autónoma de Barcelona.
Research Consortium
- Swedish Foundation For Strategic Research (SSF): “New Biomarkers in Early Diagnosis and Treatment of Alzheimer´s disease” . Agneta Nordberg (PI), Eric Westman, Christer Halldin Karolinska Institutet (KI), Hans Ågren Royal Institute of Technology ( KTH), Bengt Långström Uppsala University (UU)
- Swedish Research Council (VR) Dementia Platform, “Proteinopathies in neurodegenerative diseases –new imaging biomarkers and targets for new drugs”, Agneta Nordberg (PI), Christer Halldin , Per Svenningsson KI, Hans Ågren KTH, Bengt Långström UU
- Innovative Medicines Initiative (IMI) AMYPAD, “Amyloid imaging to prevent Alzheimer´s disease”. Project coordinator Frederik Barkhof, University of Amsterdam. PI Stockholm( KI) Agneta Nordberg.
Research support
With special thanks to the following donors for the support they provide or earlier provided to our research:
- Swedish Research Council (VR)
- Swedish Foundation for Strategic Research (SSF)
- Swedish Brain Foundation
- Swedish Alzheimer foundation
- The Stockholm County Council and Karolinska Institutet regional agreement of medical training and clinical research (ALF)
- Karolinska institutet Foundations
- The Dementia Foundation
- Foundation for Old Servants
- Gun and Bertil Stohne´s Foundation
- Sigurd and Elsa Goljes Memorial
- Loo and Hans Osterman Foundation
- Tore Nilsson Foundation
- KI Foundation for Geriatric Disease
- KTH-SLL grant
- Axel Linders Foundation
- Åke Wiberg Foundation
- The EU FW7 large-scale integrating project INMIND (http://www.unimuenster.de/INMIND)
- JPND
- IMI AMYPAD