Our research
Few medical inventions have affected and saved so many lives as vaccines against infectious diseases. The challenges we are facing today with developing effective vaccines to several of the world’s most serious infectious diseases (e.g. HIV-1/AIDS, malaria, tuberculosis), new pandemics as well as designing therapeutic vaccines to tumors and/or allergies require a much more intimate understanding of the mechanisms dictating vaccine responses.
The development of vaccines based on nanoparticles or mRNA encoding for pathogen antigens has emerged as a new era in vaccinology. The potential impact of these new vaccine technologies is enormous both in terms of safety and cost reduction. Yet, a lot of fundamental understanding of the vaccine: host interactions and the immune functions dictating the quality of responses after their administration is largely lacking.
Our group has had a long term focus on central questions in vaccinology related to both the early events after vaccine administration and the induction and quality of vaccine antigen-specific immunity. This includes studies of how vaccine antigen, adjuvants and mRNA vaccines interact with different cell populations at the site of injection and further disseminate in the body. Since induction of durable and high titers of antibodies with epitope breadth is a prerequisite to prevent many serious infections, several of our projects address the development of B cell responses from the germinal center reaction in lymph nodes to induction of durable and high titers of antibodies with broad specificity.
With a better understanding of how the immune system interacts with vaccines we would be better positioned to select formulations that can elicit stronger immunity, be used at lower doses or with fewer immunizations and are not associated with side effects.
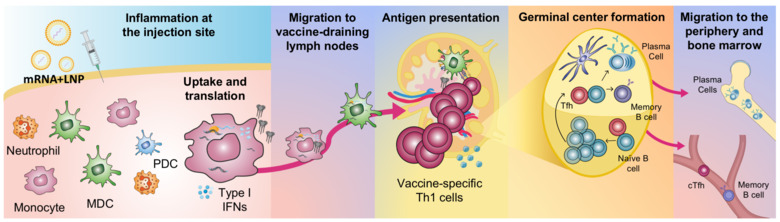
Ongoing research
In ongoing projects we investigate:
- How the early innate immune functions e.g. cell activation and antigen production after mRNA vaccine delivery influence the quality of the vaccine-specific responses.
- How antigens expressed by mRNA vaccines or on nanoparticles can increase vaccine-specific B cell and T cell immunity.
- Correlates between innate immune profiles and the induction of high-quality and durable antibody responses to licensed vaccines in human cohorts.
- How different novel adjuvants stimulate cell populations and enhance and polarize the immunity to be suitable to different infections or tumors.
Application and development of computational tools for immunological data
High-throughput technologies allow us to understand the immunological system as never seen before. However, as the data dimensionality increases, there is a need for the use of computational tools to explore and analyze these complex datasets. Our laboratory uses whole transcriptomics, deep B cell receptor sequencing, and bead-based protein arrays to decipher how our immune system is triggered upon vaccination. We have used transcriptomics to establish early transcriptional changes after vaccination in different tissues and different vaccine platforms. In addition, we are also studying antibody evolution, by integrating both antigen-specific sequencing and deep sequencing of B cell receptors to study antibody lineage development in vaccine trials. Finally, we are also developing new computational biology tools such as R packages and pipelines to help process and analyze these datasets.


