Our research
Obesity and its associated comorbidities continue to rise worldwide at an alarming rate, with severe socio-economic consequences. A deeper understanding of why overnutrition leads to disease development is urgently needed, so that improved therapeutic and preventive treatments can be developed.
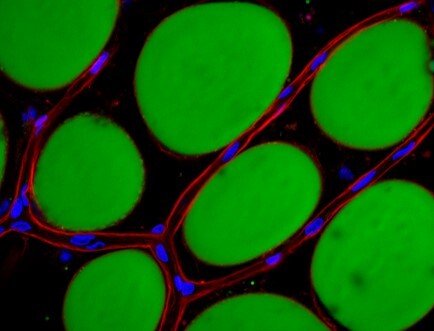
Our group studies various aspects of nutrient handling, uptake and storage in humans, with a specific focus on lipid handling by the adipose tissue, macrophage and liver. The overarching objective is to use translational approaches to elucidate pathways that, when disturbed, contribute to the development of cardiometabolic disease, with a particular focus on atherosclerosis and Type 2 Diabetes Mellitus. Our research follows three main lines.
The team of Carolina Hagberg studies lipid handling within our major lipid handling organ, the white adipose tissue. A large part of the work focuses on the crucial role of the microvasculature for adipose tissue lipid uptake and expansion, and uses these insights to, among other, create improved mechanistic in vitro research models such as the human unilocular vascularized adipocyte spheroids, HUVAS (Ioannidou et al, Journal of Physiology, 2022). The team also studies the effect of various nutrients on adipose tissue growth, and in a project led by Professor Rachel Fisher, the role of the perivascular fat found around larger vessels as contributor to the development of cardiovascular disease.
Within the Hagberg team, a study led by Ferdinand Van T Hooft focuses on characterizing novel proteins involved in triglyceride metabolism in liver and the circulation, and the relationships to known disease risk factors and makers of both metabolic and cardiovascular disease.
Lastly, the team of Professor Ewa Ehrenborg focuses their research on the interplay between lipid handling, inflammation and autophagy in relation to cardiovascular disease. Specifically, their interests center around on how lipid handling in macrophages affects inflammation and autophagy during the atherosclerosis process, something that has remained largely unknown. They also aim to identify factors/proteins in the autophagy process that are of relevance for the development of vulnerable plaques and/or progression of atherosclerosis in the vessel wall.
Together, these combined research lines cover a wide range of organs and mechanistic studies into human cardiometabolic pathophysiology, aiming to understand what promotes the emergence of disease during obesity and aging, and providing new therapeutic approaches to reduce disease burden.

