Our projects
Protecting cerebrovascular integrity after thrombolysis in acute ischemic stroke
Team leader/contact: Ingrid Nilsson, PhD (ingrid.nilsson@ki.se)
An ischemic stroke occurs when a blood clot (thrombus) occludes an artery in the brain leading to reduced oxygen and nutrient availability and eventually infarction of the brain tissue supplied by the blocked artery. Intravenous thrombolysis (clot lysis) with recombinant tissue plasminogen activator (tPA) is the gold standard treatment of acute ischemic stroke. Safety and efficacy of thrombolysis is however limited due to an increased risk of intracerebral hemorrhage. We have found that thrombolytic treatment, in both ischemic stroke patients and an experimental stroke model in mice, activates lipolytic degradation of stored lipids in adipose tissue leading to an acute elevation of circulating fatty acids in blood (Nilsson et al. 2024, BioRxiv).
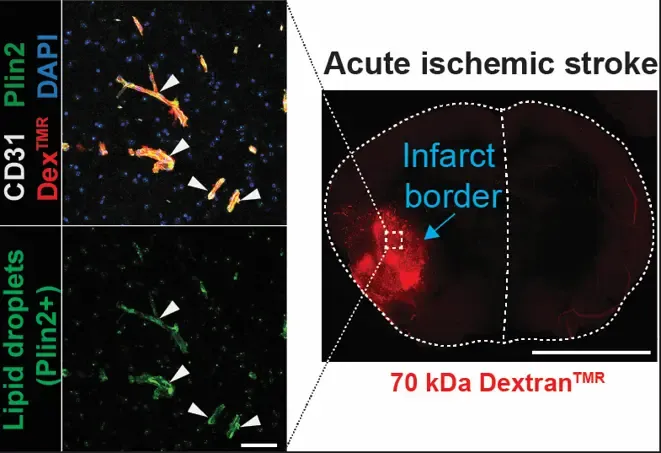
We are further exploring this novel activity of tPA in adipose tissue and the influence of elevated circulating fatty acids on cerebrovascular integrity and intracerebral hemorrhage risk. Anti-vascular endothelial growth factor-B (anti-VEGF-B) therapy successfully mitigates intracerebral hemorrhage risk and improves the efficacy of tPA treatment by reducing lipid-induced vascular stress and enhancing endothelial resilience during reperfusion. These findings highlight anti-VEGF-B therapy as a promising co-treatment together with thrombolysis that could significantly influence clinical practices.
The role of PDGF-CC/PDGFRα signaling in modulating blood-brain barrier integrity and fibrotic scar formation after ischemic stroke
Team leader/contact: Linda Fredriksson, PhD (linda.fredriksson@ki.se)
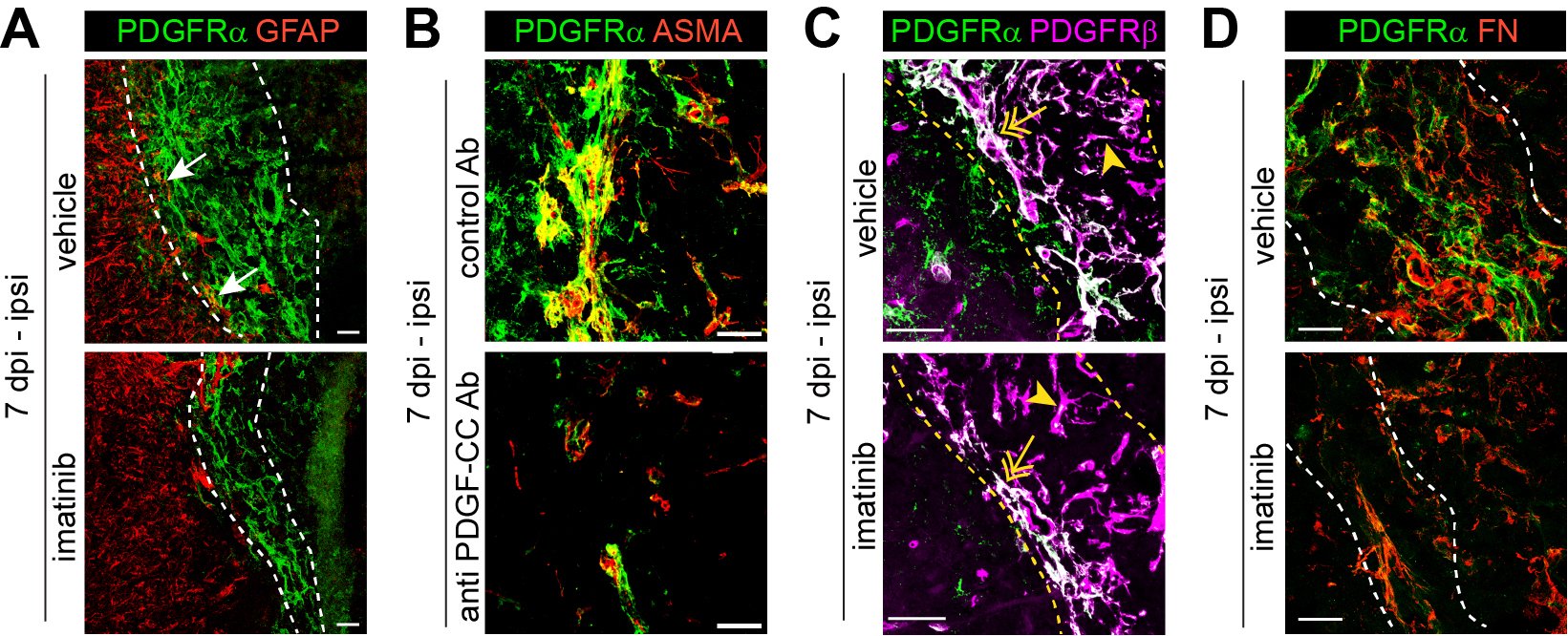
Our research focuses on the role of PDGFRα in ischemic stroke, particularly its impact on blood-brain barrier (BBB) integrity and fibrosis. BBB breakdown is a hallmark of ischemic stroke and is linked to poor clinical prognosis. We have shown that activation of PDGF-CC, the ligand of PDGFRα, by tissue plasminogen activator (tPA) disrupts BBB integrity during ischemic stroke, suggesting that PDGFRα signaling modulation could be a promising therapeutic strategy (Su et al. 2008, Nature Medicine). Based on these findings, a phase II clinical trial evaluated the safety and efficacy of imatinib, an inhibitor of PDGFRα and other receptor tyrosine kinases, in acute ischemic stroke patients treated with intravenous thrombolysis (IVT). The trial demonstrated promising results (Wahlgren et al. 2017, Journal of Internal Medicine) and will be followed by a phase III clinical trial. Furthermore, we recently demonstrated that inhibiting PDGFRα reduces myofibroblast expansion in the fibrotic scar during the chronic phase of ischemic stroke, significantly improving recovery—even when inhibition was initiated 24 hours post-occlusion (Protzmann et al. 2025, Journal of Clinical Investigations). These findings suggest that targeting PDGFRα could serve as a viable post-acute treatment strategy for stroke recovery, addressing a critical gap where current interventions are limited to rehabilitation therapy. Current projects in the lab investigate the use of a monoclonal anti-PDGF-CC antibody (Li et al. 2018, PLoS One; Zeitelhofer et al. 2018, PLoS One) as a targeted approach to block PDGFRα signaling in ischemic stroke, as well as potential progenitors of PDGFRα+ myofibroblasts contributing to fibrotic scar formation.
Intravital imaging of the cerebrovasculature in ischemic stroke
Team leader/contact: Linda Fredriksson, PhD (linda.fredriksson@ki.se)
In our lab, we have set up advanced two-photon imaging of photothrombotic ischemic stroke through a chronic cranial window to track BBB integrity, hemodynamic changes and cellular responses of cells in the neurovascular unit (NVU) in real time (Protzmann et al. 2024, Fluids and Barriers of the CNS). We employ a variety of reporter mouse lines (labeling endothelial cells in the vessel wall or other cells of the NVU) and intravenously (i.v.) administered fluorescent dyes in combination with genetic and pharmacologic inhibition of PDGF-CC/PDGFRα signaling to shed light on the blood-brain barrier after ischemic stroke. This technology allows us to longitudinally elucidate and correlate key mechanisms of vascular dysfunction from the early critical hours to the days/weeks after the infarct. A current project in the lab focuses on in-depth studies of intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (tPA) under different conditions by visualizing it in real time at an unprecedented resolution.
Video: Real-time two-photon imaging of IVT with tPA in an endothelial reporter mouse (Claudin5-GFP) that was subjected to experimental ischemic stroke. Blood flow was visualized with the fluorescent tracer TRITC70 (red). The occluded middle cerebral artery (MCA) can be seen in the center of the video. tPA was administered i.v. 35 min after vessel occlusion. Blood clot lysis and re-establishment of blood flow can be seen 145 min post ischemia.
Mechanisms of endothelial nutrient transport
Team leader/contact: Ingrid Nilsson, PhD (ingrid.nilsson@ki.se)
Endothelial cells line the inner wall of all blood vessels and take an active part in the delivery of nutrients, primarily fatty acids and glucose, to the underlying tissues.
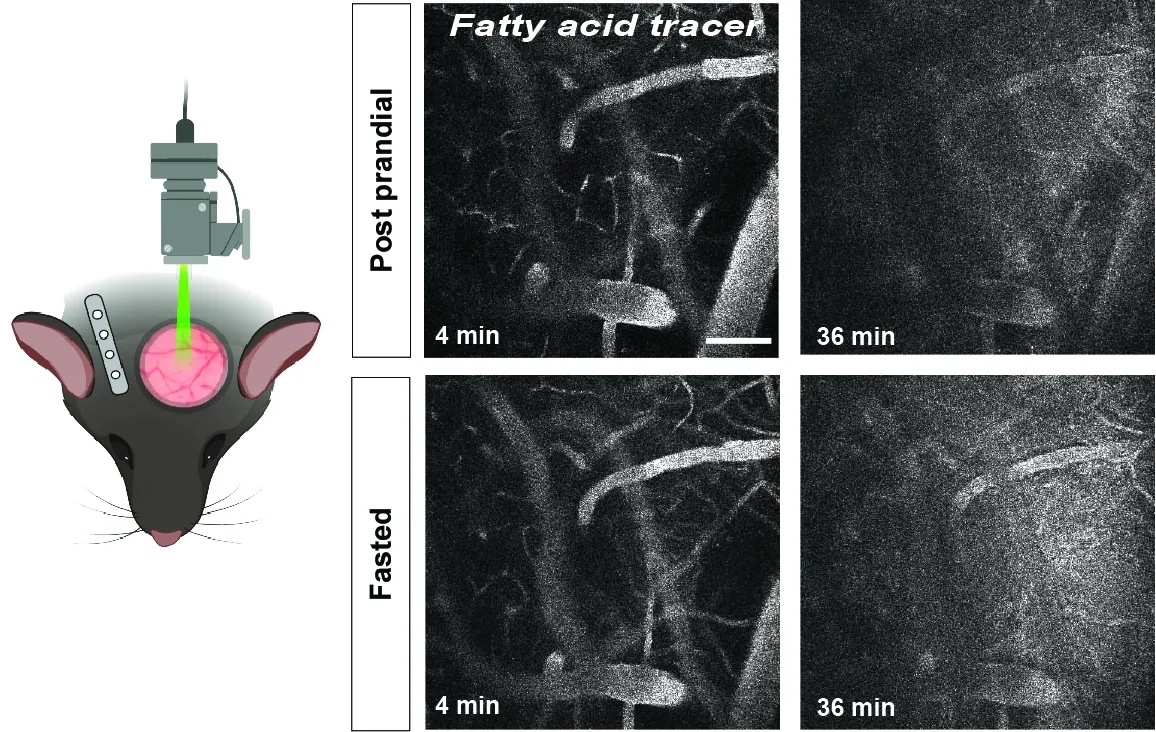
This is a highly regulated process, and we have previously shown that tissues can instruct the local vascular bed to upregulate endothelial fatty acid uptake from blood by increasing the expression of vascular endothelial growth factor-B (VEGF-B) that acts via its receptors, VEGFR1 and neuropilin-1, on endothelial cells. By combining biochemistry and molecular biology techniques, our current research aims to discover novel mechanisms that link trans-endothelial fatty acid transport to intra-endothelial lipid droplet metabolism and cellular bioenergetics. We are especially interested in studying the process of cerebrovascular fatty acid uptake and its consequences for brain health.
Linking adipose lipid dynamics to kidney lipotoxicity via vascular growth factors in diabetic kidney disease
Team leader/contact: Annelie Falkevall, PhD (Annelie.falkevall@ki.se) and Erika Folestad, PhD (Erika.folestad@ki.se)
Our research explores how vascular growth factors regulate adipose lipid dynamics and contribute to kidney lipotoxicity and Diabetic Kidney Disease (DKD). Vascular Endothelial Growth Factors (VEGFs) and Platelet-Derived Growth Factors (PDGFs) are key regulators of white adipose tissue (WAT) structure and function. Specifcially, we study how VEGF-B and PDGF-C signaling in WAT affects lipid mobilization and kidney health. Current clinical markers for DKD progression, such as albuminuria and eGFR, lack sensitivity and specificity. Evidence links dysregulated lipid handling in WAT to increased circulating free fatty acids (FAs), ectopic lipid deposition, and organ dysfunction. In DKD, this manifests as kidney lipotoxicity, fibrosis, and renal impairment. We hypothesize that vascular growth factors contribute to this adipose–renal axis.
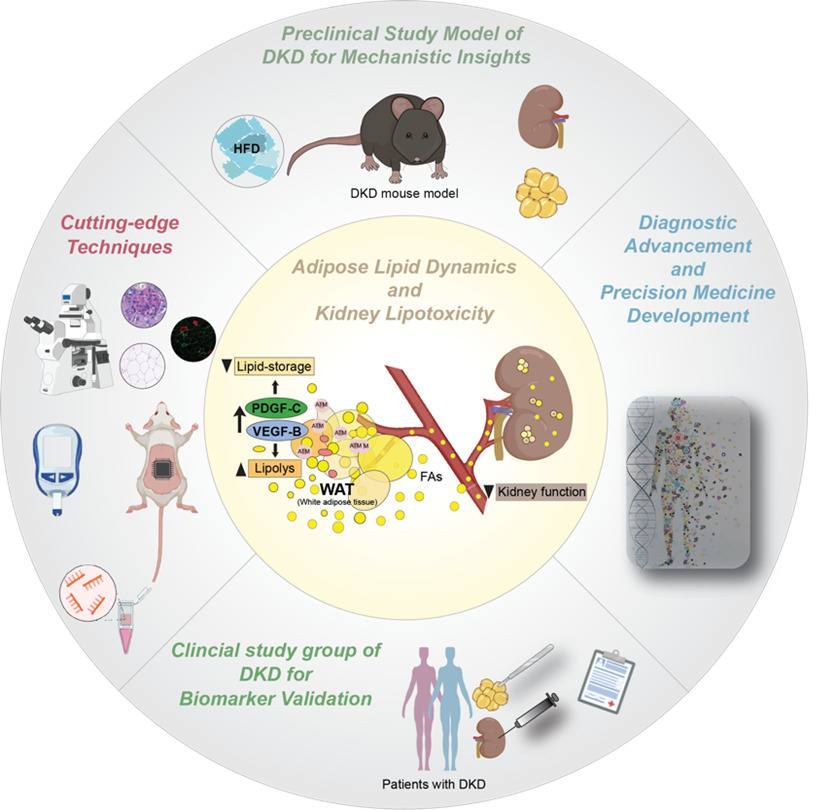
In our recent publication (Folestad et al., Kidney International, 2024), we demonstrated that VEGF-B signaling is upregulated in WAT from DKD patients, correlating with lipid accumulation in the kidney and dysfunction. Using transgenic mouse models, we showed that adipocyte-specific VEGF-B modulation affects WAT lipolysis, fatty acid release, and DKD progression, linking WAT signaling to renal damage.
Building on these findings, we aim to validate VEGF-B and PDGF-C as biomarkers in WAT biopsies from DKD patients and test their roles in kidney dysfunction using experimental models. Our goal is to develop a minimally invasive WAT-based diagnostic tool for early detection and stratification of DKD patients. In this project, we are collaborating with Prof. Peter Stenvinkel, MD, PhD, and Prof. Anders Thorell, MD, PhD, at Karolinska Institutet.
Disrupting adipose-driven lipotoxicity: VEGF-B and PDGF-C as therapeutic targets in MASLD-Driven Liver Cancer
Team leader/contact: Annelie Falkevall, PhD (Annelie.falkevall@ki.se) and Erika Folestad, PhD (Erika.folestad@ki.se)
Our research investigates how vascular growth factors regulate lipid mobilization from white adipose tissue (WAT) and contribute to hepatic lipotoxicity and hepatocellular carcinoma (HCC) in metabolic-dysfunction-associated steatotic liver disease (MASLD). We focus on VEGF-B and PDGF-C signaling in WAT and how these pathways promote liver steatosis, fibrosis, and tumor development. In insulin resistance, dysfunctional WAT enhances lipolysis, releasing free fatty acids that accumulate in the liver and drive disease progression. However, mechanisms linking adipose lipid flux to liver tumorigenesis remain poorly understood.
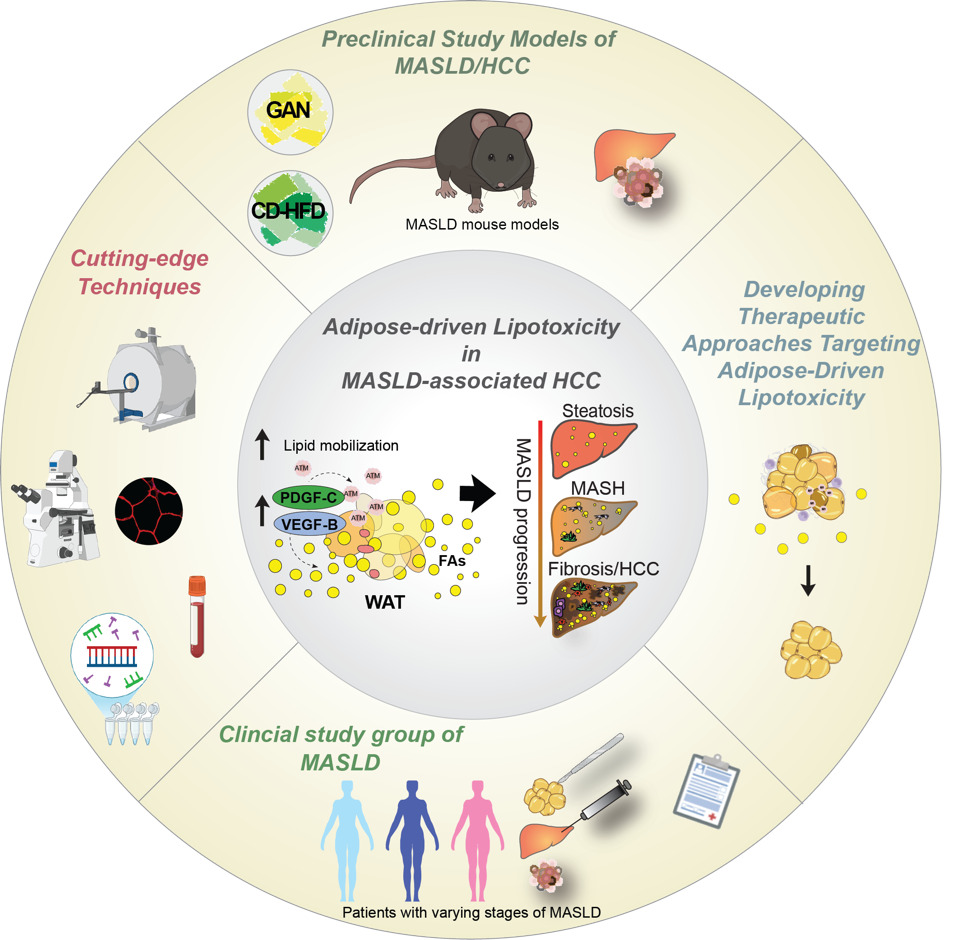
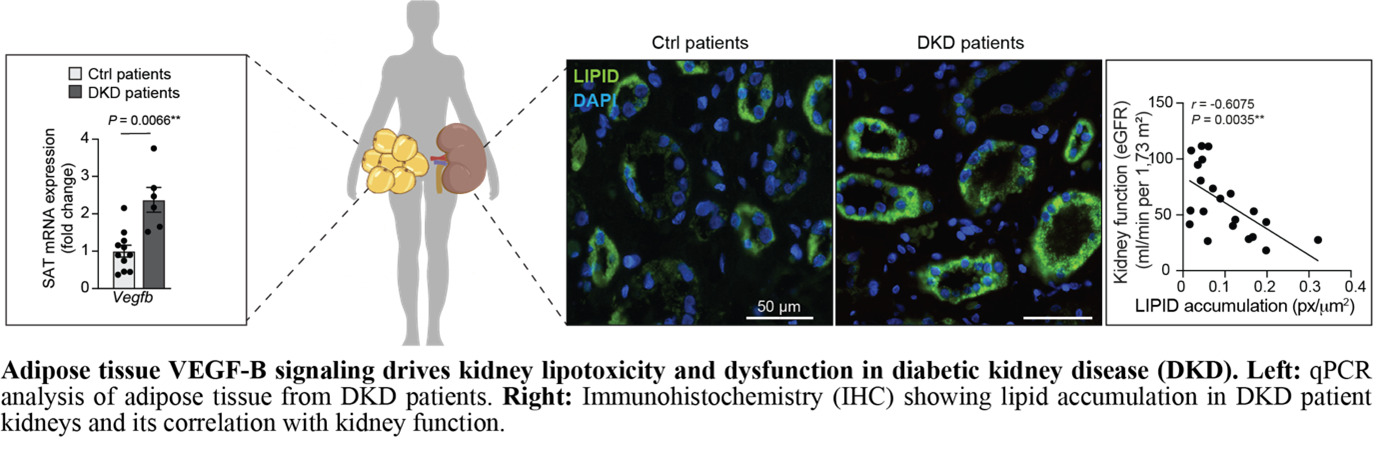
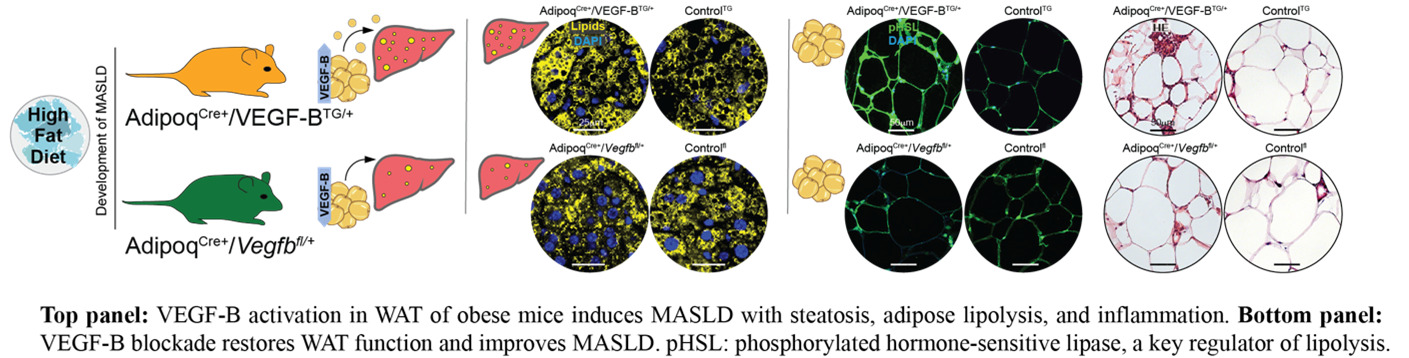
In our recent Journal of Hepatology publication (Falkevall et al., 2023), we showed that VEGF-B signaling in WAT drives hepatic steatosis via hormone-sensitive lipase activation, and that its inhibition prevents MASLD in diabetic mice. PDGF-C, enriched in adipose macrophages, promotes WAT expansion, liver fibrosis, and HCC in preclinical models.
Current projects test whether genetic or pharmacological inhibition of lipid mobilization reduces hepatic lipotoxicity and prevents MASLD-related HCC, using mouse models, different diets (Gubra Amylin NASH diet (GAN), choline-deficient high-fat diet (CD-HFD), and human WAT and liver biopsies. By uncovering how adipose tissue contributes to liver cancer, this research may lay the foundation for therapies targeting adipose-driven lipotoxicity. We will expand these findings to investigate how lipid mobilization contributes to other obesity- and metabolism-associated cancers. In this project we are collaborating with Prof. Hannes Hagström, MD, PhD; Assoc. Prof. Niklas Björkström, MD, PhD; and Prof. Per Stål, MD, PhD at Karolinska Institutet.
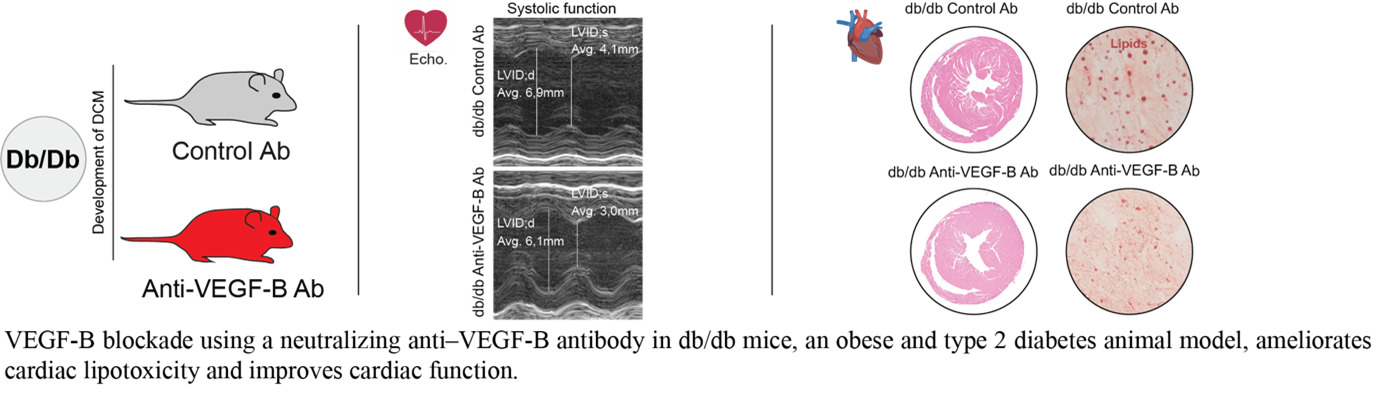
Vascular growth factor signaling in adipose-heart crosstalk and Diabetic cardiomyopathy
Team leader/contact: Annelie Falkevall, PhD (Annelie.falkevall@ki.se) and Erika Folestad, PhD (Erika.folestad@ki.se)
Our research explores how vascular growth factors regulate lipid mobilization and contribute to cardiac lipotoxicity, fibrosis, and diabetic cardiomyopathy (DCM). We investigate how VEGF-B and PDGF-C signaling affect white adipose tissue (WAT) lipid storage and heart function in type 2 diabetes (T2DM). Elevated free fatty acids from insulin-resistant WAT accumulate in the heart, driving lipotoxic damage, yet the pathways linking systemic lipid mobilization to DCM remain poorly understood.
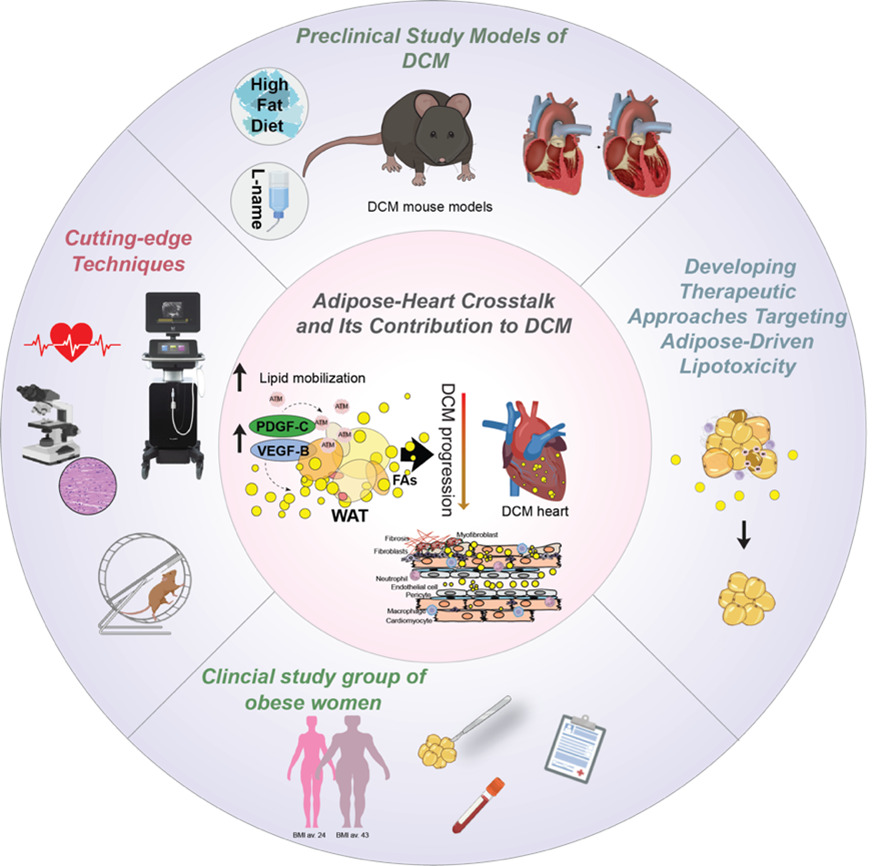
Building on our studies (Falkevall et al., Journal of Hepatology, 2023; Folestad et al., Molecular Aspects of Medicine, 2018), we have shown that VEGF-B regulates WAT lipolysis and ectopic lipid accumulation in metabolically active tissues, and that PDGF-C, a potent fibrogenic factor, is implicated in interstitial fibrosis—a hallmark of DCM.
Current projects assess how VEGF-B and PDGF-C blockade affect cardiac function and fibrosis in T2DM mouse models. Using genetically engineered mice with tissue-specific deletion or overexpression in adipocytes, immune cells, and cardiomyocytes, we evaluate local versus systemic effects on cardiac lipid accumulation, remodeling, and function. In this project, we are collaborating with Assoc. Prof. Ljubica Matic, Docent, PhD; Assoc. Prof. Daniel Andersson, MD, PhD; and Gianluigi Pironti, PhD at Karolinska Institutet.