Research description
The Pete Williams Lab uses the eye as a model of the central nervous system to elucidate early mechanisms of neurodegeneration. We utilize modern transcriptomic and molecular tools to delicately dissect pathways pertaining to early aging and neurodegenerative disease mechanisms and to identify potential therapeutic targets which we then test and verify in animal and cell models of neurodegeneration. We work with clinicians to develop novel protective strategies that will benefit human health, aging, and disease.
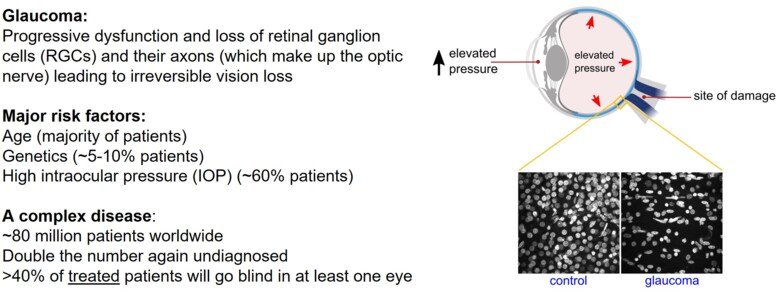
Glaucoma is a complex, multifactorial disease affecting an estimated 80 million people worldwide and is the leading cause of irreversible blindness. It is a significant health and economic burden at both individual and societal level. Age, genetics, and high intraocular pressure (IOP) are all considerable risk factors. Despite strategies to manage IOP, >40% of treated glaucoma patients will progress to blindness. Neuroprotective treatments for glaucoma are of great therapeutic need. The Pete Williams Lab is developing new neuroprotective treatments for glaucoma, from bench-to-bedside.
The overarching theme of the Pete Williams Lab is to explore how bioenergetic insufficiency drives neurodegeneration and to identify novel neuroprotective therapies based on these data
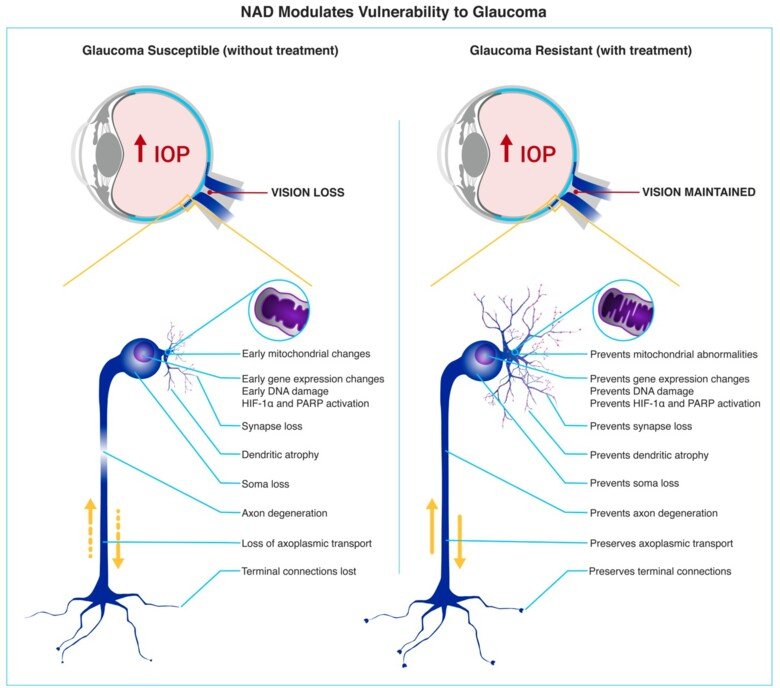
Glaucoma is characterized by the progressive dysfunction and loss of retinal ganglion cells (RGCs). RGCs are the output neurons of the retina, the axons of which become the optic nerve before integrating with key visual centers in the brain. Axon and dendritic degeneration are core components of glaucomatous neurodegeneration. RGCs sit on a metabolic knife-edge during times of stress that may be exacerbated by aging, genetic risk, and elevated IOP. During these periods, the viability of RGCs is reliant on mitochondria and supportive glia to maintain homeostasis and bioenergetic needs. Emerging research suggests that a systemic vulnerability to mitochondrial and metabolic abnormalities exists in glaucoma patients. Genomic analysis has demonstrated increased mitochondrial DNA content and a spectrum of mitochondrial DNA mutations in glaucoma patients. These abnormalities are also present in leukocytes, suggesting a systemic susceptibility to metabolic defects. Such systemic susceptibility is expected to increase glaucoma susceptibility with age.
Only strategies to lower IOP have been translated to the clinic and these therapeutics do not treat the neurodegenerative component of glaucoma. Patient adherence is variable and too many patients are subject to surgical interventions because of progressive damage. In addition, many patients are refractory to pressure lowering treatments and progress to blindness despite low pressures. So far, search for a treatment that targets RGCs and arrests progression, has a low side effect profile, and is cost-effective has been unsuccessful. Neuroprotective treatments for glaucoma are of great therapeutic need. Our research explores how this bioenergetic insufficiency drives neurodegeneration and identifies novel neuroprotective therapies based on these findings.
Major Current Research Theme: Neuronal NAD+ metabolism
NAD supplementation protects from bioenergetic insufficiency in glaucoma. We have previously discovered metabolic dysfunction and mitochondrial abnormalities occurring prior to neurodegeneration in glaucoma (in glaucoma patients and animal models). A key finding of these studies identified that NAD (an essential REDOX cofactor and metabolite) declines in the retina in an age-dependent manner and renders RGCs susceptible to glaucoma-related stresses, driving neurodegeneration. Preventing NAD depletion via administration of nicotinamide (NAM; the amide of vitamin B₃; an NAD precursor) or through gene therapy (Nmnat1 or Nmnat2; terminal enzymes for NAD production) robustly protects from age-related neuronal metabolic decline and prevents glaucoma in chronic animal models. Supporting a hypothesis in which pathogenically low NAD leads to glaucoma susceptibility, glaucoma patients have been demonstrated to have systemically low levels of nicotinamide (in sera), and we, as part of a multi-national collaborative clinical trial, have demonstrated that nicotinamide administration can restore visual function in existing glaucoma patients. Our continued work with nicotinamide exemplifies the lab’s commitment to developing translational neuroprotective strategies, from bench-to-bedside.