Our research
The TRANslational Theranostics Group at Karolinska Institutet, also recognized as Tran Lab, is advancing theranostic radiopharmaceuticals for diagnostic and therapeutic applications.
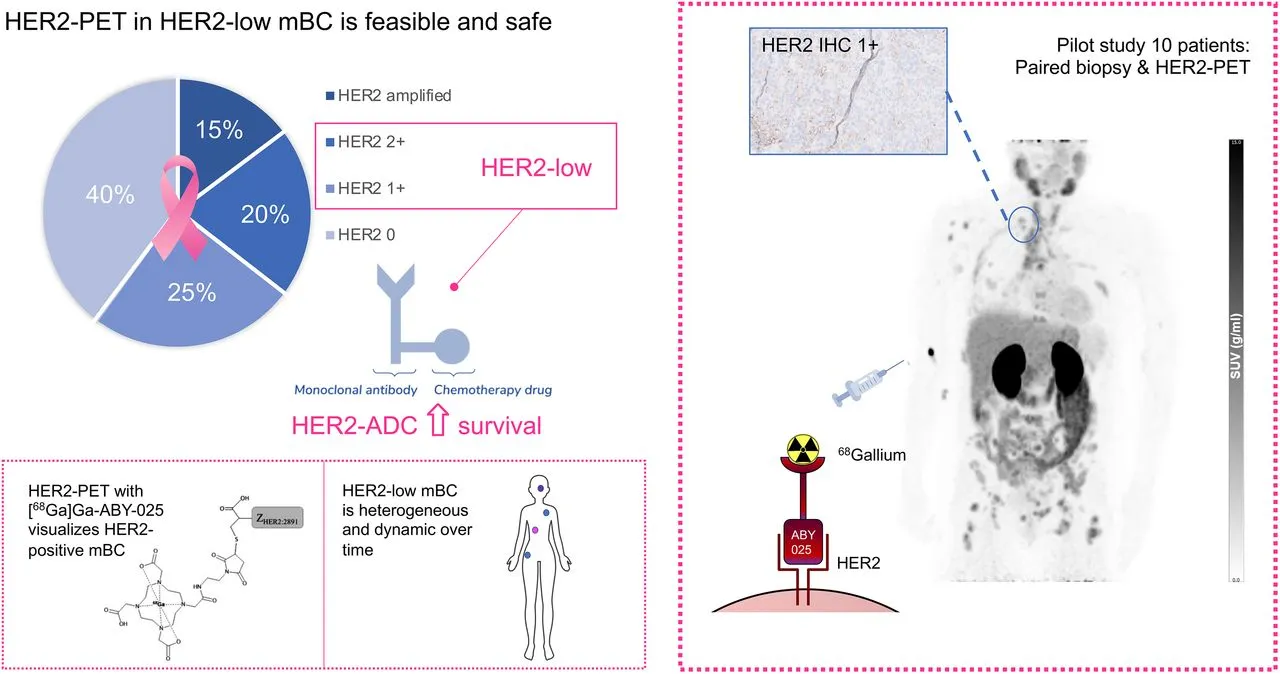
Our research focuses on the development of targeted radiopharmaceuticals for theranostics, i.e. radioactive drugs that can be used both for therapy and diagnostics. We perform specifically:
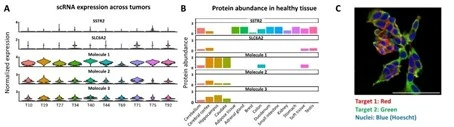
- Discovery and identification of novel targets for cancer treatment
- Drug screening for new drug candidates.
- Advancement of non-conventional radiometals for diagnostics and therapy
- Development of radiolabelling methods
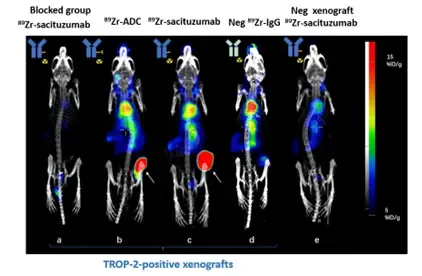
- In vitro evaluation in cell cultures and in vivo evaluations in animal models
Our research group is a diverse and multidisciplinary team at the Department of Oncology and Pathology at the Karolinska Institutet. Many of our members are also clinically active at the Karolinska University Hospital, within the Karolinska Radiopharmacy and the Department of Medical Radiation Physics and Nuclear Medicine.
Methodologies and Infrastructure
The TRANslational Theranostics Group is based at the Dept of Oncology and Pathology, and Dept of Karolinska Radiopharmacy, both located in Bioclinicum, Solna.
The Karolinska Radiopharmacy at Karolinska University Hospital has a new-built state-of-the-art radiochemistry facility, equipped with:
- 2 cyclotrons (PETrace 800, GE)
- In total 32 research and GMP hotcells (Comecer)
- Several synthesis modules (GE, Trasis, E&Z, ITM etc)
- New analytical instrumentations (Agilent, LabLogic etc)
We have access to the solid target on one of the cyclotrons and dedicated radiochemistry labs for the preclinical radiometal and radiochemistry development. We collaborate closely with research leaders within target identification and drug screening and utilizing crucial national infrastructure at Scilifelab and the core facilities at KI (such as nanoPET/CT at KERIC).