B Cells and Autoinflammation
Systemic lupus erythematosus, SLE, is a complex autoimmune disease and unfortunately to this day without cure. The goal with the treatment is to minimize organ damage and flares of disease, as well as comorbidities such as atherosclerosis. A major problem however, is that when the patients are diagnosed at the clinic, there is already irreversible organ damage. Still, autoantibodies occur years before disease onset and thus finding B cell directed treatments and novel clinical markers could stop the disease at an early stage.
In our group we focus research on systemic immunological mechanisms originating in the spleen. One rationale for this is that the spleen contains our largest pool of immune cells as well as specific subtypes and has evolved to sense and balance systemic immunity. Even though the focus is inflammatory mechanisms in SLE, the findings from my group will be central for many inflammatory diseases and conditions. An example of this is that we have found that macrophage polarization, driven by specific pattern recognition receptors, can be targeted for immunotherapy for cancer using an antibody. We are currently moving forward to test this novel treatment and are generating anti-human antibodies that could be used in the clinic.
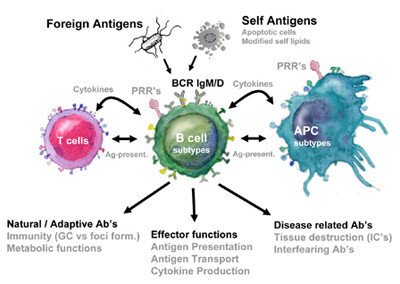
Current research areas
We carry out research in the areas of autoimmunity, allergy, cardiovascular disease, cancer and infection.
Autoimmunity
To study the activation and recruitment of B cells in innate autoreactive responses we study the B cell responses to self-antigens using syngeneic apoptotic cells (mimicking the autoimmune disease SLE) and other inflammatory stimuli. In this project we ask which APC and innate or adaptive T cell reactivity that are involved in regulating autoimmune B cell recruitment. We also investigate B cell recruitment in primary immunodeficiencies leading to autoimmunity such as APS I were mutations in the AIRE gene leads to defect tolerance affecting B cell activation.
Allergy
Even though much is known about the T cell dependent events leading to IgE production, little is known about the early events in the selection of B cells that produce IgE. We study this in models of both asthma and eczema to evaluate B cell activation. In one of these projects we study Atopic eczema (AE)which is a chronic relapsing disease often beginning early in childhood. In the context of B cell activation one subgroup of AE shares some features with autoimmune diseases since IgE that is being produced is partly against self-structures and this self-reactivity also correlate with disease severity. We currently investigate questions with regard to B cell recruitment and regulation in both models for allergy as well as in patient cohorts.
Cardiovascular disease
Our studies on the recruitment of B cells in SLE using apoptotic cells as antigen have many connections to atherosclerosis. First, SLE patients have greatly increased risk of atherosclerosis and second many of the receptors we are studying are also involved in fat metabolism. For these reasons we compare B cell activation in atherosclerosis with SLE and try to find ways to harness the positive effect that B cells have in this disease to design novel B cell directed treatments.
Cancer
Metastatic cancer develops primarily at secondary sites by interaction with stromal cells within a supporting extra cellular matrix. Within the mixture of stromal cells, macrophages have an important role in producing factors that both lead to and support cancer growth. As such, tumor associated macrophages (TAM) and the related myeloid-derived suppressor cells (MDSCs) represent the major inflammatory component of the stroma in many tumors and increased numbers of these cells are associated with poor prognosis. In our studies on B cell regulation by specific macrophage subtypes we have found that specific regulators of TAM expressed antigens could potentially be used to affect the inflammatory response. Thus we aim to study the interaction between macrophages and B cells in cancer stroma.
Infection
The early innate responsiveness of the immune system is not only important for quick immune responses to pathogens but also to initiate and shape the subsequent adaptive immune response. Recognition of pathogen-associated molecules by pattern recognition receptors such as Toll-like receptors (TLR) and NOD like receptors (NOD) by antigen presenting cells (APC) is followed by internalization of the microbe and antigen presentation on MHC molecules. As of recent a molecular platform that integrates different innate signals has been defined and named the inflammasome. When assembled upon activation the inflammasome cleave and activates cytokines such as IL-1, IL-18 and IL-33. Some of these cytokines can also be found to be elevated in autoimmune and allergic diseases. In this project we want to define the role of these signals in recruitment and regulation of B cell responses in the context of self vs non-self.
Methods currently used
Models for allergy, cancer, autoimmunity and atherosclerosis, flow cytometry and sorting including live signaling, CBA, ELISA, ELISPOT, multiphoton intravital microscopy, confocal laser scanning microscopy, TIRF (Total internal reflection fluorescence microscopy), cell culture (primary cells and cell lines), Antibody production and genetics/molecular biology techniques.
