Our body consists of ~50% of skeletal muscles, which are crucial for our ability to breathe and move. Skeletal muscle is also important for energy homeostasis; its a primary site for glucose uptake and glycogen storage and a reservoir for amino acids stored as protein. Muscle dysfunction, comprising muscle weakness and altered metabolism, is a common comorbidity in many non-communicable diseases, such as type 2 diabetes, peripheral artery disease, rheumatoid arthritis, and cancer, as well as normal ageing. Muscle dysfunction can reduce both the ability to work and the quality of life for afflicted patients and is considered to accelerate mortality.
Research projects
Our lab focuses on deciphering the molecular mechanisms that contributes to disease-induced muscle dysfunction and identifying novel therapeutic interventions to counteract muscle weakness. Altered Ca2+handling, free radical signaling, and mitochondrial function are key factors in the intramuscular interplay that contributes to impaired muscle function and hence central components in our research.
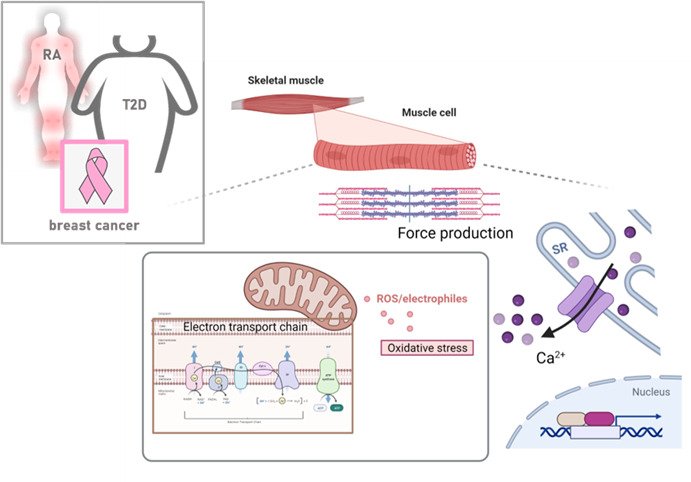
In the lab, we currently elucidating the mechanisms underpinning muscle dysfunction associated with rheumatoid arthritis (RA), obesity, type 2 diabetes (T2D) and peripheral artery disease as well as breast cancer. We are also interested in basic muscle biology questions and currently investigating how modulators if mitochondria alter the electron transport chain and the overall capacity to generate energy.
Methods
In our lab we have created an interdisciplinary and translational niche for making ground-breaking research on the causes of muscle function and dysfunction. This includes human patients and healthy subjects, as well as preclinical models, which are used together with functional analyses (e.g. live imaging, muscle contraction, cellular respirometry), ultrastructural analyses (e.g. electron microscopy, near super-resolution imaging), in vivo assessments (e.g. muscle strength, indirect calorimetry) and multi-omics analyses. This approach allows us to enhance our knowledge about disease-induced muscle dysfunction, as well as bridge gaps between disciplines, foster creativity and innovation.
 Photo: Creative Commons CC0
Photo: Creative Commons CC0Make a donation to our research
Your support means a lot to the success of research. This allows us to go further in our efforts to improve human health through research and education.

