Investigating hepatocyte and liver macrophage remodeling in NAFLD
Liver is the major organ of energy metabolism. The metabolic pathways in the liver are tightly controlled to balance its anabolic and catabolic activities. Under conditions of over-nutrition this balance is disrupted, which promotes lipid accumulation in the hepatocytes to form a pathological phenotype of steatosis. Liver steatosis can further proceed to more severe stages of non-alcoholic steatohepatitis (NASH), fibrosis and cirrhosis, which can ultimately develop into liver cancer. So far there is no clinically approved pharmaceutic therapy to reverse the process, largely due to limited understanding of the molecular events underlying the disease development.
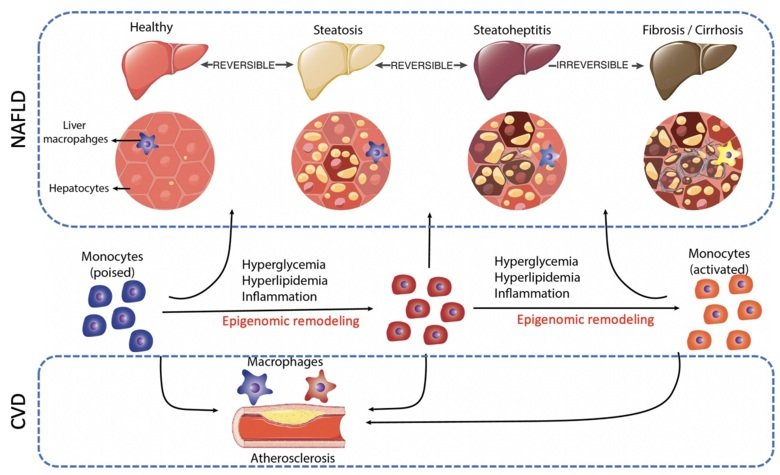
It is generally accepted that dysregulated liver lipid metabolism and inflammation are two crucial mechanisms involved in NAFLD development, although the transcription network and the related epigenome remodeling events remain largely unclarified. Data mining in liver disease patient cohorts allowed us to identify several candidate epigenomic modulators that are correlated with NAFLD phenotypes. Our ongoing efforts attempt to validate these candidates and to explore their mechanism of action through a variety of methodologies, including tissue-specific mouse models and next-generation sequencing approaches.
Unraveling the lipid-regulated monocyte activation mechanisms in atherosclerosis
The high burden of CVD is attributable to the increasing incidence of atherosclerosis. Atherosclerosis is initiated by endothelium dysfunction, followed by immune cell (including monocytes) infiltration into the vascular intima to induce forming of plaques. Activation of monocytes by pathological factors (i.e. obesity, hypercholesterolemia and hyperglycemia) serves as the key mechanism to promote the disease development. Moreover, such immunological activation exhibits ‘memories’ which cause disease relapse and drug resistance.
In collaboration with the clinical researchers at the Karolinska University Hospital, we plan to apply established epigenome analysis techniques in a large and well-characterized Swedish cohort of patients with lipid disorders. The major aim is to understand how an excess of lipids in the circulation alters the monocyte epigenome, particularly at enhancer regions, and modulates their metabolic activation, memory and atherogenic properties.
Looking for a Postdoc, Master or PhD Project?
We always welcome motivated Master students, PhD students and postdocs who have a relevant background and a keen interest in our research. Please contact rongrong.fan@ki.se for potential projects and vacancies
