Our research
Aside from blood cancer cells, several cell lines from solid cancers respond to DHODH inhibitors in culture. However, the results in vivo are less impressive. One possible reason for this is that the levels of uridine in plasma can supplement UMP production and therefore rescue cells from DHODH inhibition.
After identifying a number of potent and specific inhibitors of DHODH, our current research is devoted to finding strategies to improve their therapeutic index for cancer treatment. This is performed by identifying agents that rescue normal cells from DHODH blockage (including T cells and their antitumour function), determining which tumor types are hypersensitive to inhibition of this key enzyme and by elucidating the reasons for this hypersensitivity at the molecular level.
Discovery and elucidation of the mechanisms of action of tumour selective compounds
Activating p53 has long been considered as an attractive strategy to treat cancer. Since 2004, the most specific approach to activate wildtype p53 without damaging DNA is to use inhibitors of mdm2 with peptides or small molecules such as nutlin3 (Vassilev et al., Science, 2004). However, and in spite of the enormous efforts of academia and industry, mdm2 inhibitors have yet to deliver in the clinic. This slow progress may be due to lack of selectivity for cancer cells or because many cancer cell types tend to undergo a reversible cell cycle arrest. An additional concern is that mdm2 inhibitors such as nutlin3 can lead a significant proportion of cells to accumulate in G2. This effect, which also occurs in cultures of normal cells such as primary fibroblasts could have undesired consequences. Indeed, as demonstrated by others and confirmed by us, when the mdm2 inhibitor is removed G2 cells can enter S phase leading to the appearance of 8N cells and increasing the risk of genome instability.
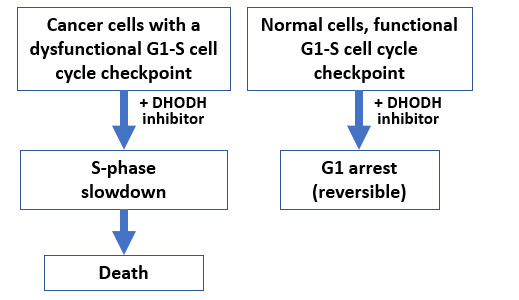
Although less selective for the p53 pathway than mdm2 inhibitors, numerous other small molecules activating wild type p53 have been described in our lab. Of these, a large number happen to be inhibitors of the enzyme dihydroorotate dehydrogenase DHODH, a mitochondrial enzyme coupled to the electron transport chain that is involved in the de novo synthesis of uridine 5’-monophosphate (UMP) and therefore, of all pyrimidine ribonucleotides. As such, DHODH inhibitors target highly proliferating cells (including activated T cells) and are therefore used in the clinic for the control of autoimmune diseases and are in clinical trial as antivirals. With regards to cancer, it is well established that inhibition of UMP synthesis can lead to the accumulation of cancer cells in S phase, a very vulnerable stage of the cell cycle and where p53 activation may cause cell death more easily. In addition, DHODH inhibitors can promote differentiation of leukemic cells, which is why several DHODH inhibitors have entered clinical trials for acute myeloid leukemia.
News archive
Support our research
 Photo: Chokniti Khongchum
Photo: Chokniti KhongchumMake a donation to our research at MTC
Your support means a lot to our success. This allows us to go further in our efforts to improve human health through research and education.
Read here how you can make a donation via Swish.
