Our research
We know that 7 simple steps help protecting our heart and brain from myocardial infarction and stroke: stop smoking, exercise regularly, have a healthy diet, reduce blood cholesterol and sugar, lose weight, control blood pressure. Even so myocardial infarction and stroke still happens in middle-aged men and women who follow this advice. This has dramatic consequences for the single individual and society. The focus of our research is to understand what we miss to reduce the burden of these diseases. To this end we use a so-called molecular epidemiological approach: in large population consisting of adult individuals at different ages we try to identify novel proteins or blood components that differ between individuals whose heart and brain stay healthy and those who suffer a myocardial infarction or a stroke and eventually die. Implementation of this research brings to a deep understanding of the factors that increase the individual risk but also to the discovery of novel targets for treatment and prevention of diseases of the heart and vessels.
Endotypes can explain the inflammatory response in atherosclerosis
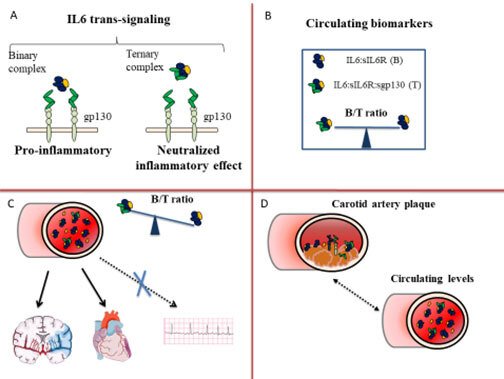
We have recently identified a novel biomarker that mirrors the inflammatory component of a circulating citokyne, Interleukin 6 (IL6) . Figure 1 summarizes the rationale behind our studies and the main results. IL6 exerts a pro-inflammatory and pro-atherosclerotic effect through the binding to a soluble receptor, sIL6R, a signalling moiety known as IL6 trans-signalling (Figure 1, Panel A). To avoid a systemic inflammatory response, the binary complex (B) (IL6:sIL6R) is antagonized by a soluble antagonist, the glycoprotein sgp130. The three molecules form a circulating ternary complex (T). At steady state, the binary and ternary complexes are balanced in the circulation (Figure 1, Panel B). In inflammatory conditions, the binary complex exceeds the buffer capacity of sgp130 and a pro-inflammatory state supervenes [12]. To estimate the CV risk associated with an excess of the pro-inflammatory binary complex, we have calculated the B/T ratio, i.e. the ratio between the binary and ternary complexes (Figure 1, Panel B).
A high B/T ratio corresponds to a relative excess of the pro-inflammatory binary complex. In the cohort of 60 years old men and women from Stockholm (60YO) (n=4232) a high B/T ratio associated with an increased risk of CHD and ischemic stroke (Figure 1, Panel C) and improved prediction and reclassification measures of the risk of both CHD and ischemic stroke (Ziegler L et al Cardiovasc Res 2019), but not of other common CV disease as atrial fibrillation which also increases the risk of ischemic stroke through an atherosclerosis independent meachanism (Ziegler L et al BMC Neurology 2021). In particular, the B/T ratio improved prediction of ischemic stroke in study participants otherwise classified as at low-intermediate CV risk according to LDL-cholesterol levels and Framingham Risk Score (FRS)≤20% (Ziegler L et al Eur J Prev Cardiol 2020). Furthermore, all the components of the B/T ratio were expressed in human carotid atherosclerotic plaques in the BiKE study (Ziegler L et al Vascular Medicine 2021) and showed a moderate correlation with circulating levels of IL6 and sIL6R (Figure 1, Panel D).
2. Novel clinical and biological markers of risk for stroke and major bleeding in patients with atrial fibrillation
We have established a large cohort of elderly patients with atrial fibrillation (AF) (n=2943) the Atrial fibrillation: risk and benefits of anti-coagulation (Carebbean)-elderly (Ehrlinder H et al International J Cardiology Heart and Vasculature 2020) to identify thrombosis/bleeding clinical risk profiles. AF patients represent a group at very high cardiovascular risk, because of AF and because age per se is a hallmark of cardiovascular risk. Aging is associated with a chronic inflammatory state that creates a milieu favourable to ASCVD. We have collected clinical, biochemical and imaging data on all AF patients (aged 75–104) who have initiated an anticoagulant treatment. We follow them up for ischemic and bleeding complications before and after initiation of anticoagulant treatment. In the next future we are going to collect blood samples before and after the start of the treatment with anticoagulants to identify molecular biomarkers related to hemostasis, inflammation and angiogenesis that can help us to improve the clinical characterization of these patients.
At present, we are analysing how loss of muscular mass which represents a marker for chronic inflammation affects the risk of future ASCVD and bleeding, the two major causes of death in this group of patients.
3. Getting fit to fight cardiovascular diseases in rural Uganda: the Iganga-Mayuge cardio-pulmonary study
Cardiovascular diseases are rapidly increasing in Uganda. Malnutrition during childhood, the globalization of junk food, the aging population with its burden of comorbidities and chronic infectious diseases create a unique setting for the development of cardiovascular diseases in Uganda. The figure below summarizes the epidemiological transition from infectious to cardiovascular diseases in Uganda: while infectious diseases are still prevalent and are a common cause of death, cardiovascular diseases rise and cause progressively more deaths in the population. Infections can affect the occurrence of cardiovascular diseases in multiple ways: HIV and other chronic infectious diseases as well as the treatment against them are associated with a chronic low grade inflammation that increases the life time risk of cardiovascular diseases.
The pattern of cardiovascular risk factors in Uganda is largely unknown and most of the existing data rely on extrapolation from other sub-Saharan regions.
To this exent, we are establishing a longitudinal cohort in a rural area in eastern Uganda districts of Iganga and Mayuge, 120 kilometres east of Kampala, the capital city of Uganda. (please see the small red circle on the Ugandan map on the left). Karolinska Institutet and Makerere University (Uganda) have established the Iganga-Mayuge population-based Health Demographic Surveillance Site (IMHDSS) in 2004. Interviews are conducted every year and the demographic characteristics of this population are constantly updated. In particular, care is taken to update inhabitants for each one of the 17000 household, number of births and deaths as well as possible causes of death.
We have planned and recently started to link the demographic surveillance with a health screening of the population with special focus on young adults (25-35 years of age). We collect demographic, clinical and anthropometric data. In addition a large and complete biobank has been established. This long term project has a local and a global perspective. In the local setting of Uganda, the project is designed to build capacity, charachterize the cardiovascular risk with clinical and molecular data and provide care where possible. On a global perspective, undermining the risk factors of cardiovascular disease in Uganda where chronic inflammation is highly prevalent and other more traditional risk factors (like smoking) less prevalent can provide us with new knowledge and new directives to improve prevention of cardiovascular disease in patients with chronic inflammatory and autoimmune diseases.
