Our research
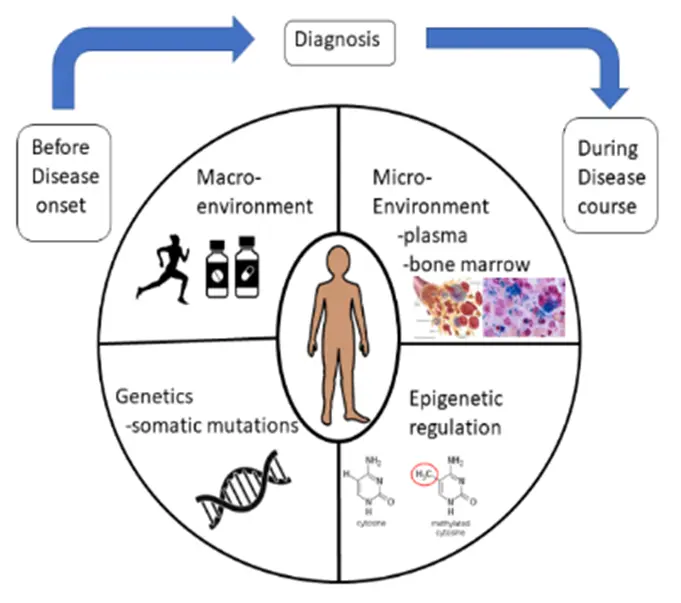
Chronic myeloid malignancies are a diverse group of hematopoietic stem cell diseases with a varying prognosis, with the common feature of having a propensity to progression/transformation to acute myeloid leukemia. Our mission is to a) understand the role of chronic inflammation and microenvironment in disease onset, b) understand how genetic, epigenetic, environment and other factors contribute to disease onset from a pre-disease state and to disease progression and prognosis, c) identify targetable factors responsible for the transition to overt disease, with the ultimate goal to d) keep these malignancies in a pre-malignant chronic state and avoid overt disease outbreak. For systemic mastocytosis, we also study the cell of origin and how this impacts the disease course of indolent (with a normal life expectancy) or advanced (with a life expectancy of 2-4 years).