Our research
Allergies are a public health issue that affects over 60 million people in Europe, and especially the prevalence of food allergy is increasing. Development of allergy is affected by different factors like hereditary conditions, method of delivery, exposure to allergens, microorganisms and other environmental factors during pregnancy and childhood.
Within the program Allergy Development and Prevention Trials (ADAPT) studies aimed at different perspectives of allergic diseases (epidemiology, diagnostics, treatment, basic research) are performed; e.g. diagnostics of parental IgE profiles, influence of pre- and post-natal allergen exposure, investigation of the impact of the immune system during pregnancy, birth and the neonatal period, various treatment strategies, and technical developments for diagnostics and monitoring during different phases of allergy development. The results obtained in this program are expected to improve diagnostics, predict and reduce allergy development in children and provide new treatment strategies for food allergic children.
Introduction
Allergies are a public health issue that affects over 60 million people in Europe, and especially the prevalence of food allergy is increasing. Food induced allergic reactions vary in severity from mild oral itching to life threatening anaphylaxis, sometimes even within the same patient. Food allergies negatively affect the quality of life for the affected children and their families. Even though the majority of young children who suffer from food allergies to milk, egg and wheat outgrow that specific type of allergy they will still be at risk of later developing other allergic diseases.
Allergy development is multifactorial but heredity and exposure to different factors like allergens, microorganisms and other environmental factors have been given the greatest importance. The time from conception until the baby is one year old is regarded as the time when chronic diseases, including allergies, are established. Health and risk factors for allergy development have previously focused on mode of delivery, diet during pregnancy, the introduction of allergenic foods early in life, intestinal flora and the exposure to animals, drugs and pollution.
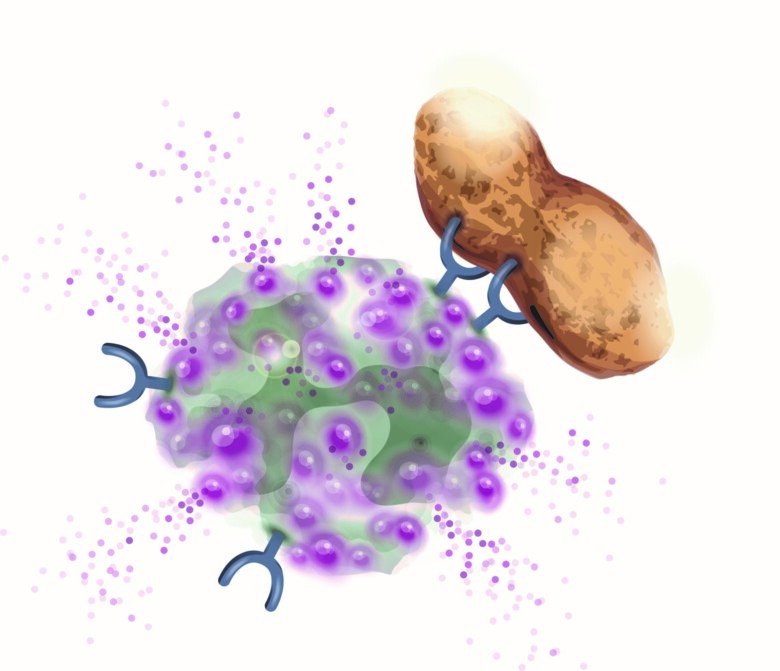
IgE-mediated allergy depends on the individual's propensity to form IgE antibodies (IgE-ab) against different allergens e.g. from food and pollen. IgE-ab binds to the surface of mast cells and basophils and subsequent allergen-exposure activates these cells leading to a release of mediators which cause allergic symptoms (Figure 1).
The diagnostic tools are evolving and today it is possible to analyse IgE-ab against individual components (proteins) of an allergen i.e. component based diagnostics (CRD). CRD gives a high specificity but only show IgE-sensitisation and not necessary true allergy. The diagnostic accuracy has also been improved by cellular in vitro methods. We have developed a method where basophils stimulated in vitro with allergens enables analysis of basophil allergen sensitivity, CD-sens. CD-sens has been shown to significantly correlate with clinical allergic reactions which makes it a valuable alternative to complex in vivo allergen challenges in the airways and orally, and has also been used to monitor treatment effect of anti-IgE (omalizumab) and allergen specific immunotherapy (ASIT).
Today only few treatments of food allergies are available and children with food allergy are mainly encouraged to avoid the food in question. With oral immunotherapy (OIT), very small amounts of the allergen are given in slowly increasing doses until a maintenance dose is reached. Studies have so far shown that OIT leads to desensitisation, but it is still unclear whether long-term tolerance is achieved. Allergic reactions, both mild and severe have been a major problem in these studies.
Projects
Development of allergy may be considered as a journey that consists of phases where various factors affects; hereditary conditions, environmental factors during pregnancy, method of delivery, exposure to allergens and environmental factors during the neonatal period and during the child's upbringing. The program “Allergy Development and Prevention Trials (ADAPT)” four cornerstones include studies aimed from different perspectives (epidemiology, diagnostics, treatment, basic research) to generate new knowledge about diagnostics and treatment during the different phases of allergy development (Figure 2).
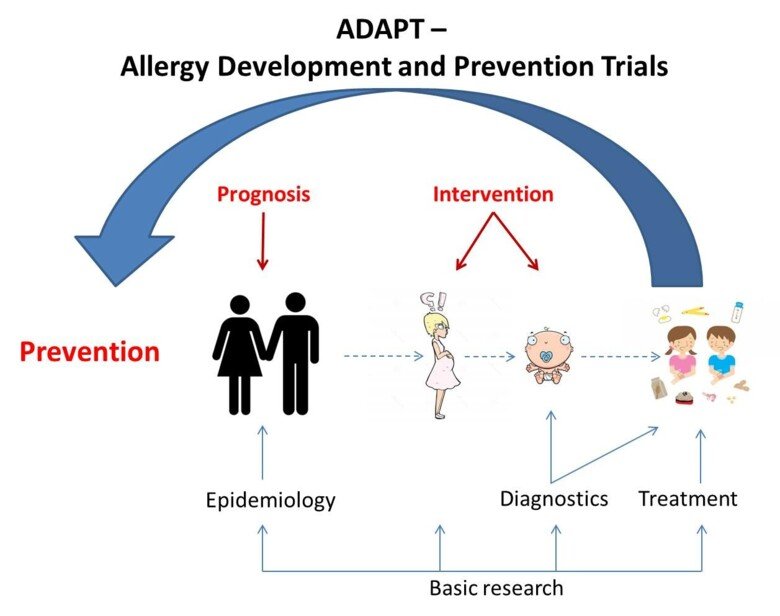
- Epidemiology – Follow up two existing multi-generation cohorts to identify markers that might predict allergy development, and to investigate whether diet during and postnatal periods affect allergy development.
- Diagnostics - Through clinical, immunological and genetic studies combined with innovative technology generate information to improve diagnostics, long term prognosis and monitoring of food allergic children.
- Treatment - In various treatment studies identify immunologic and genetic factors that might predict treatment outcome and generate new clinical individualized treatment strategies.
- Basic research - Identifying disease driving mechanisms with focus on IgE and the role of basophils during pregnancy and birth, and the development of allergy or tolerance.
We have shown that CD-sens is a useful complement for complex allergen challenges in vivo with good correlations between CD-sens and SPT-titration, bronchial-, nasal-, and oral- challenge. CD-sens can also be used to monitor treatment with anti-IgE (omalizumab, XolairÒ) and specific immunotherapy. CD-sens with flow cytometric detection is since 2010 implemented both into clinical practice and research for diagnosis and monitoring of treatment efficacy.

