Our research
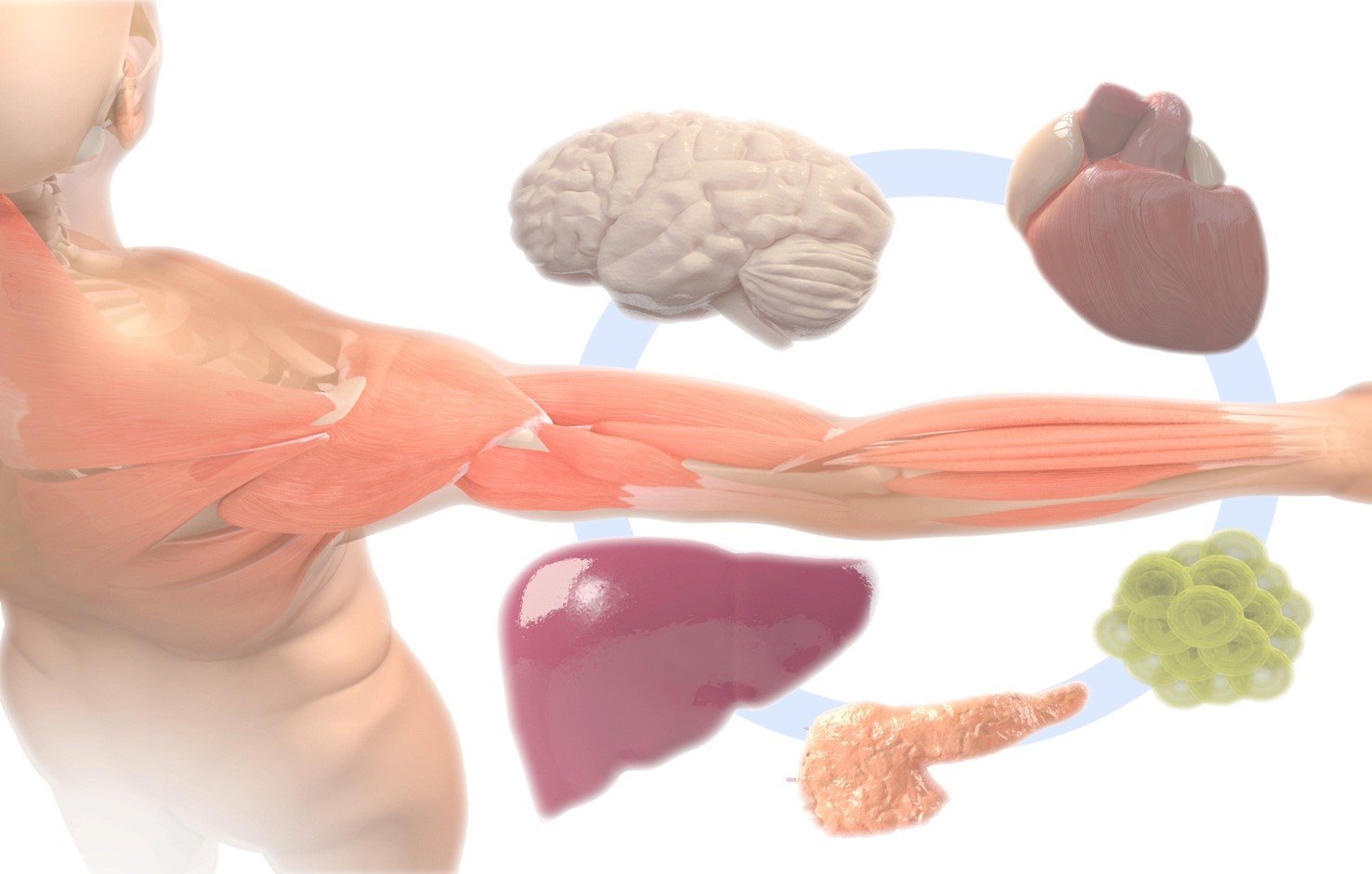
Sedentary lifestyles seem to come at a high cost to our health and have been linked to the incidence of several diseases including diabetes, obesity, cardiovascular disease, neurodegenerative diseases (such as Alzheimer’s and Parkinson’s), mood disorders (e.g. depression), and even cancer. Physical activity and skeletal muscle condition play a clear role in the prevention and treatment of these diseases. However, exercise programs are not always viable treatment options due to inherent disease characteristics such as muscle weakness, difficulty in movement, or, in particular, patient compliance.
By studying the mechanisms by which skeletal muscle adapts to different challenges and positively affects so many aspects of human health, we can learn valuable lessons that can be translated into future disease therapies.
Research Projects
The common goal of the projects being developed in our laboratory is to better understand the signal transduction and gene regulatory pathways that control skeletal muscle function in health and disease. With this knowledge, we aim at developing strategies that can be used as possible therapeutic avenues for the treatment of metabolic and degenerative diseases.
We are particularly interested in understanding how exercised or sedentary skeletal muscle can crosstalk with other organs, and how it can affect individual health and disease.
 Photo: Creative Commons CC0
Photo: Creative Commons CC0Make a donation to our research
Your support means a lot to the success of research. This allows us to go further in our efforts to improve human health through research and education.



