Project groups within the Klas Kärre Group
Projects
Human Cytomegalovirus
Human Cytomegalovirus (HCMV) is a double-stranded DNA-virus, belonging to the herpes viruses. In western countries it infects between 70-80% of the population, usually early in life. Infection under normal circumstances is asymptomatic but the virus establishes life-long latency in the host. Under certain conditions, such as immunosuppression (during cytostatic therapy, transplantation protocols or in AIDS patients), reactivation can occur and give rise to serious or even life-threatening consequences.
However the most fascinating question is how CMV escapes the host immune response for decades and how this fine-tuned equilibrium with the immune system is established. In a permanent co-evolution with the host, CMV evolved a wide array of immunoevasive strategies targeting the immune response on multiple stages. It is estimated that more than 30 viral genes serve the purpose of facilitating immune escape.
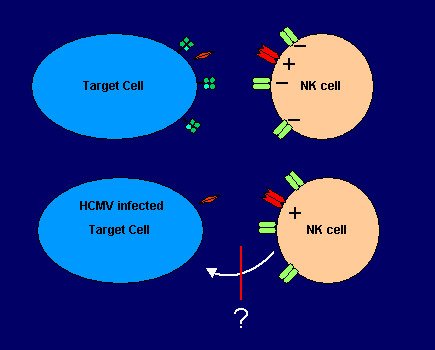
CD8+ T cells are known to be crucial for control of the virus, both during primary infection as well as during episodes of reactivation. HCMV has devoted a considerable number of genes ( e.g. US2, US3, US6 and US11, all of which have a different mode of action) to downmodulate MHC class I molecules which, in complex with CMV-derived peptides serve as recognition structures for CD8+ T cells. But the event of MHC class I downmodulation should theoretically render infected cells highly susceptible to NK-cell mediated responses (missing-self recognition, see main page). Yet several groups including our own have reported an even decreased sensitivity to NK cell cytotoxicity after infection of cells with CMV (!) This intriguing finding constitutes the fascinating core question of NK cell - CMV biology (see also cartoon).
To escape NK cells, even in face of markedly reduced MHC class I expression, the virus conceptually has two options:
- The delivery of an alternative inhibitory signal to the NK cell (e.g. by virtue of viral class I homologues)
- The interference with NK cell activating pathways.
Both alternatives are under investigation in our laboratory.

NK Cells and Cytomegalovirus
Wayne Yokoyama, Howard Hughes Medical Institute, Rheumatology Division, Department of Medicine, Washington University School of Medicine and Barnes Jewish Hospital, St. Louis, Missouri, USA.
Gerdt Riise, Department of Clinical Virology, Göteborg University, Sahlgrenska Hospital, Göteborg, Sweden.
Annika Linde, SMI/Karolinska Institutet, Stockholm, Sweden.
Cecilia Söderberg-Naucler, Cell and Molecular Immunology, Center for Molecular Medicine CMM, Karolinska Institutet, Sweden.
Eric Vivier, Centre d'Immunologie, INSERM-CNRS de Marseille Luminy, Parc Scientifique de Luminy - Case 906, 13288 Marseille Cedex 9, France.
David Cosman, Molecular Biology, AMGEN Corporation, Seattle, Washington, USA.
