Our research
Fibrotic scarring
The adult mammalian central nervous system (CNS) has a poor regenerative capacity and CNS injury most often leads to the persistent loss of body functions.
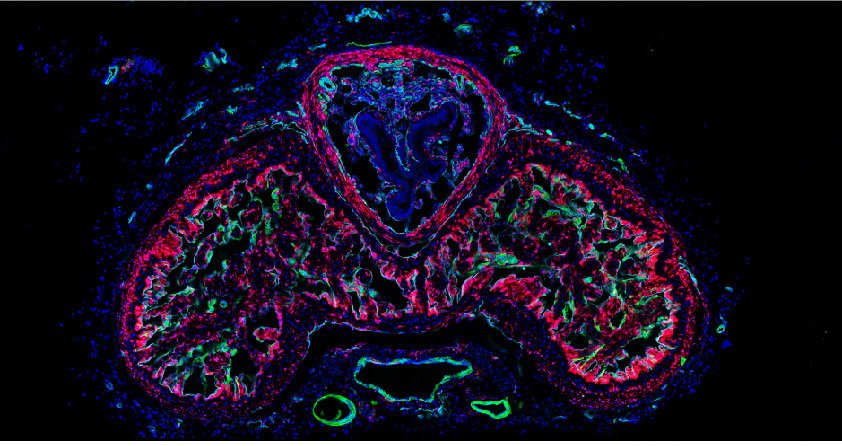
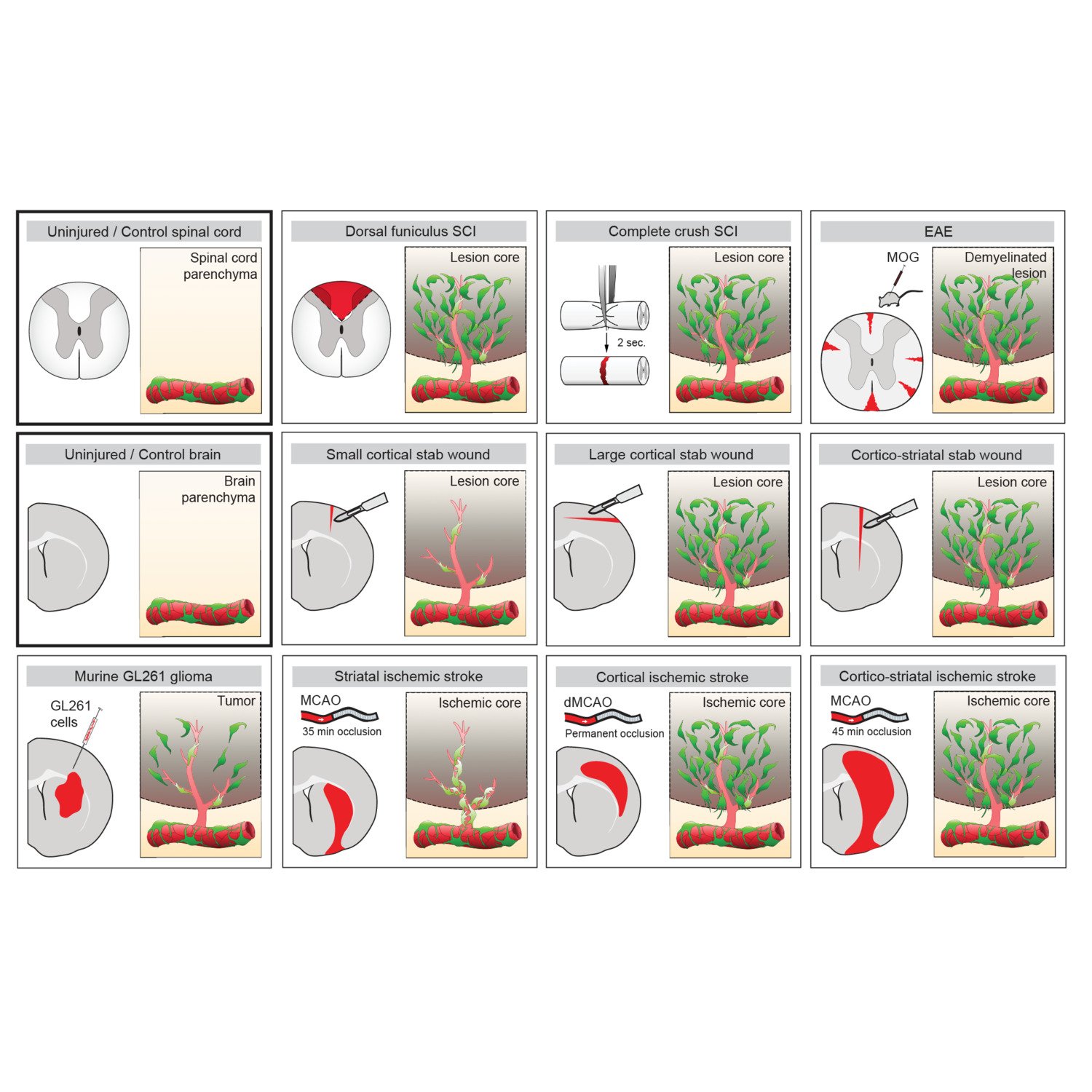
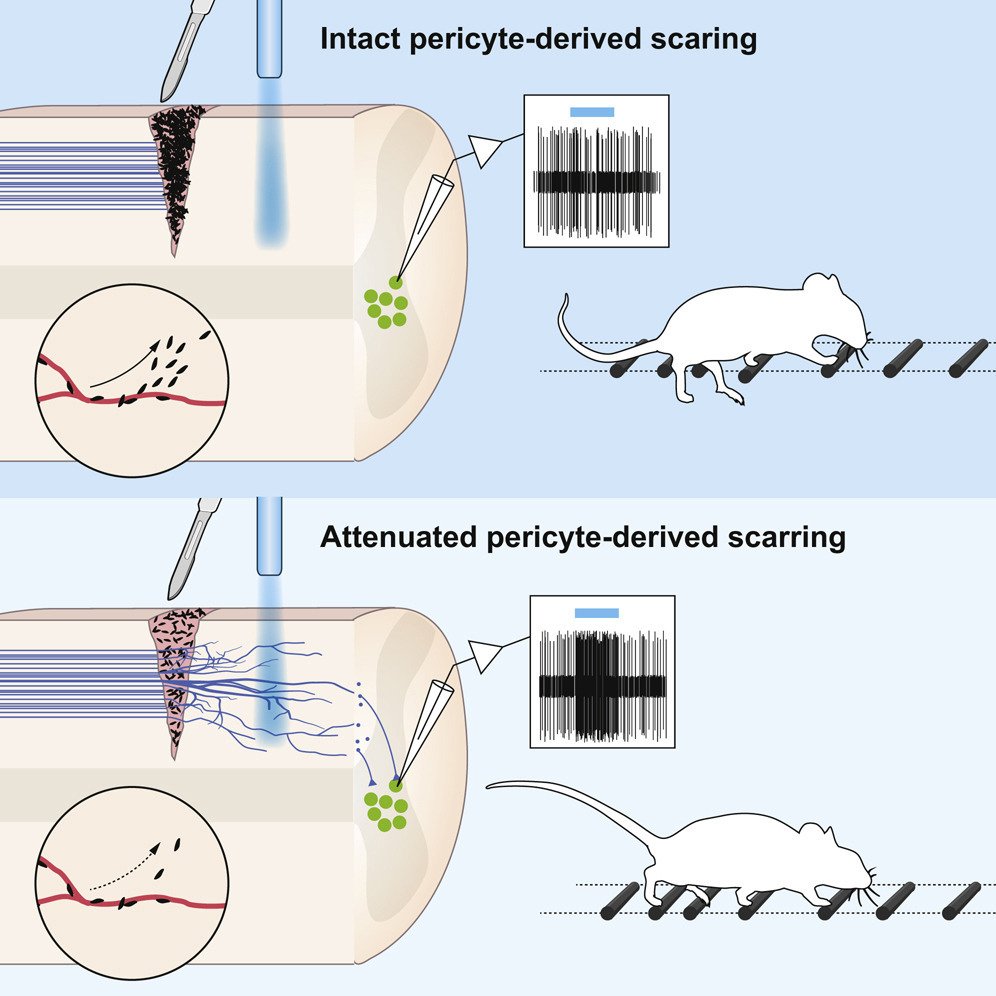
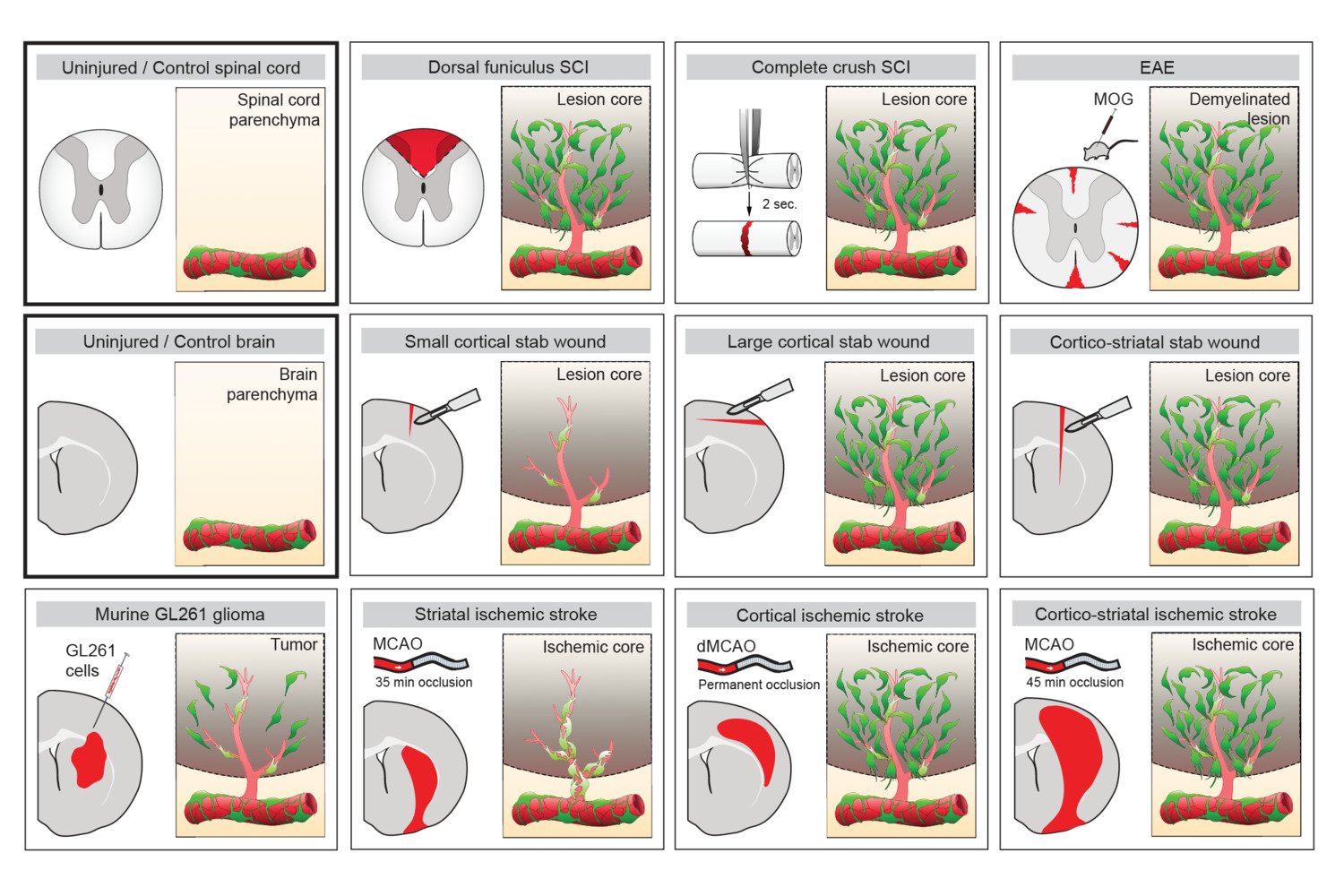
One important (biological) cause why functional deficit become permanent is the establishment of a scar that blocks regeneration of the connections between neurons (axons). In our research, we explore the origin and function of scar tissue and how the fibrotic scar component influences axonal regeneration and functional recovery after spinal cord injury. We previously discovered a specific subpopulation of cells surrounding blood vessels (perivascular cells) as the main source of fibrotic scar tissue following a variety of distinct CNS injuries. Perivascular-derived stromal fibroblasts mediate wound closure and are thus important to re-establish tissue integrity after injury, but at the same time constitute the long-term persistent fibrotic scar. We have shown that attenuation of perivascular-derived scarring led to improved regeneration of nerve fibers and functional recovery. Thus, we believe that modulation of fibrotic scarring represents a therapeutic target to treat CNS injury, which we explore further in a variety of CNS lesions.
Endogenous cell replacement
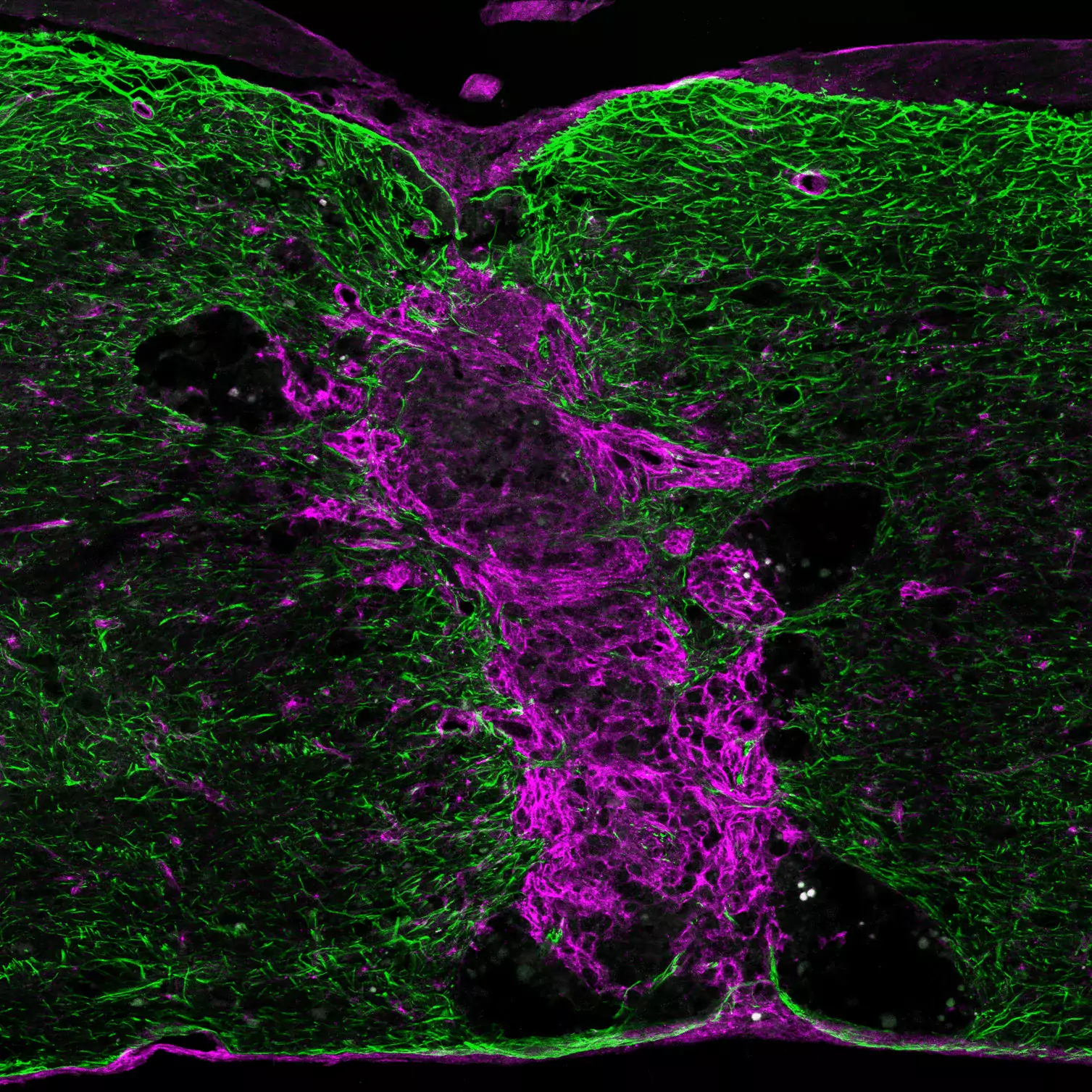
For tissue regeneration, not only scar tissue needs to be removed, but lost cells need to be replaced. We study endogenous stem / progenitor cells and reprogramming strategies to achieve this. Astrocytes have emerged as a potential source for new neurons in the adult brain. In mice, adult striatal neurogenesis can be stimulated by local damage, which recruits striatal astrocytes into a neurogenic program. We identified Notch signaling as a key pathway controlling striatal astrocyte-derived neurogenesis. Furthermore, we showed that astrocyte-derived neurons can functionally integrate into the striatal circuitry of adult mice.
Physiological function
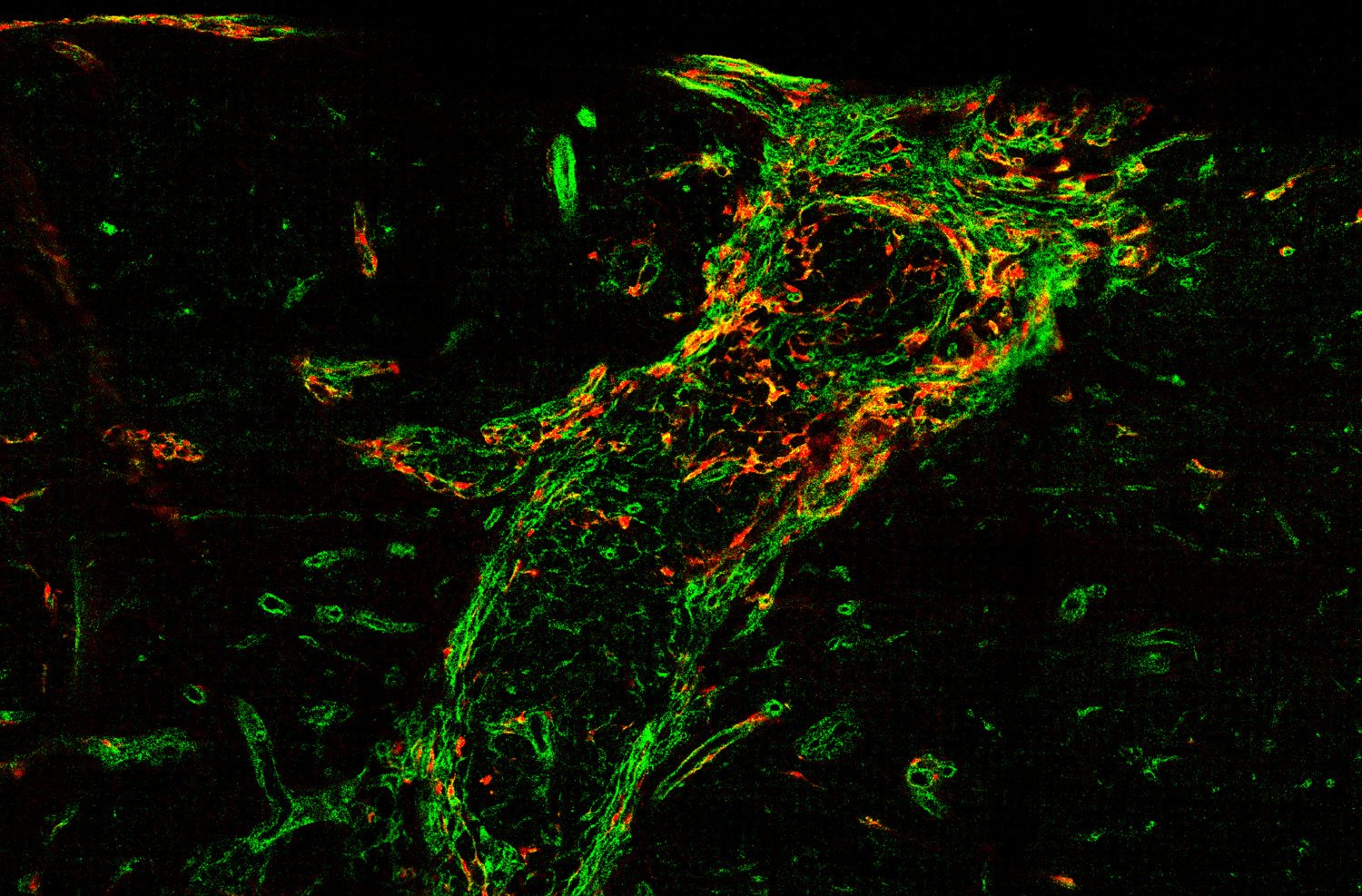
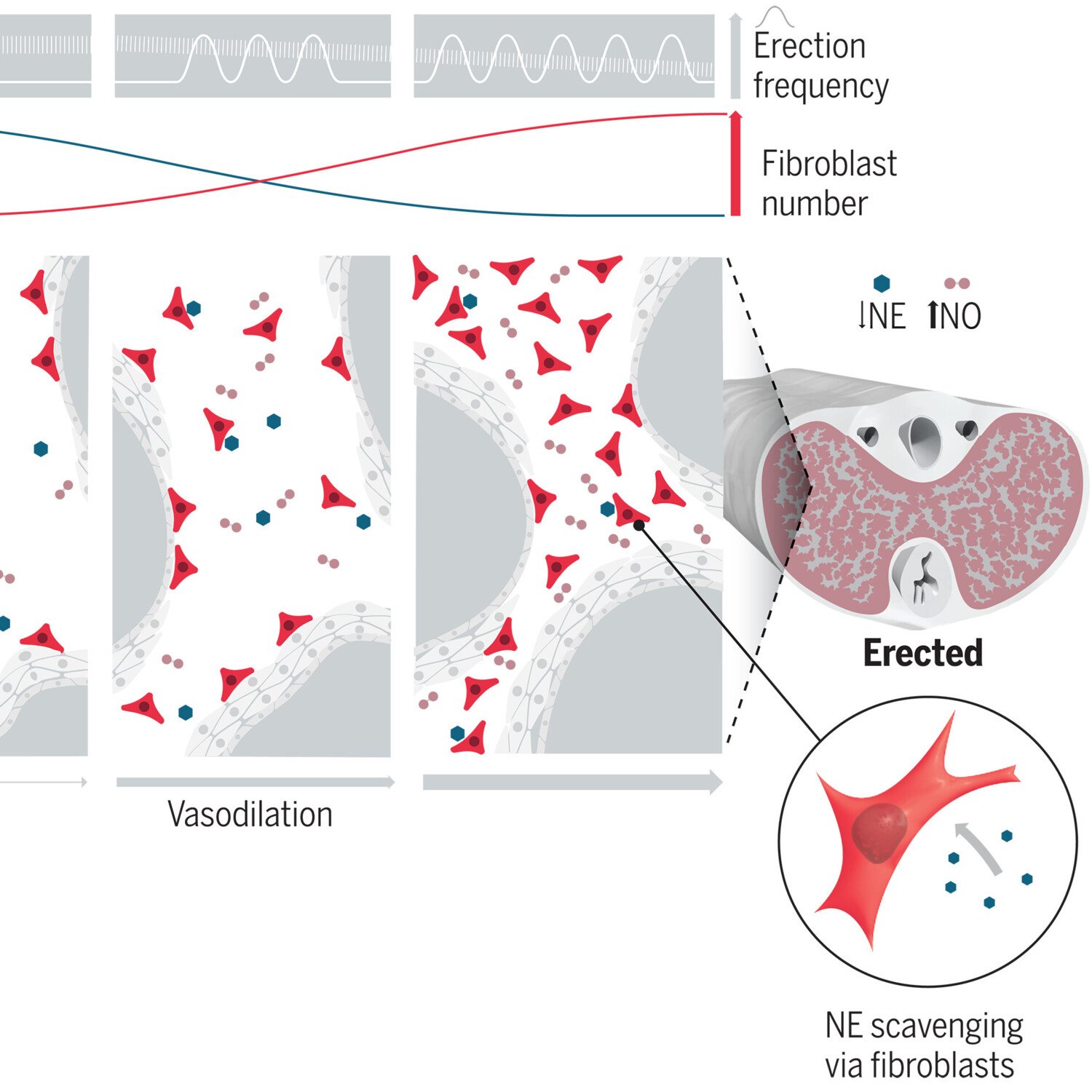
We also explore the physiological function of perivascular cells and especially perivascular fibroblasts. In some organs like the penis, perivascular fibroblasts are highly abundant. However, their physiological function is unknown. We are investigating the role of perivascular fibroblasts for penile erection.