Our Research
The knowledge gained from developmental biology research is widely applied in regenerative medicine. Thus, we hope to improve human health via discovering new fundamental ideas about how development, stem cells, and regeneration work.
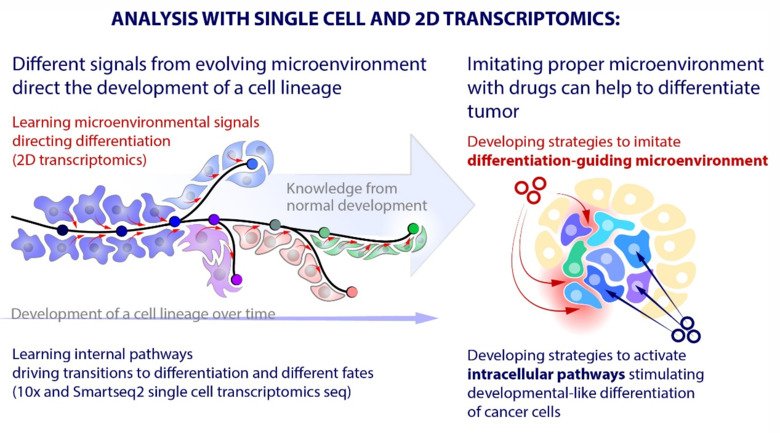
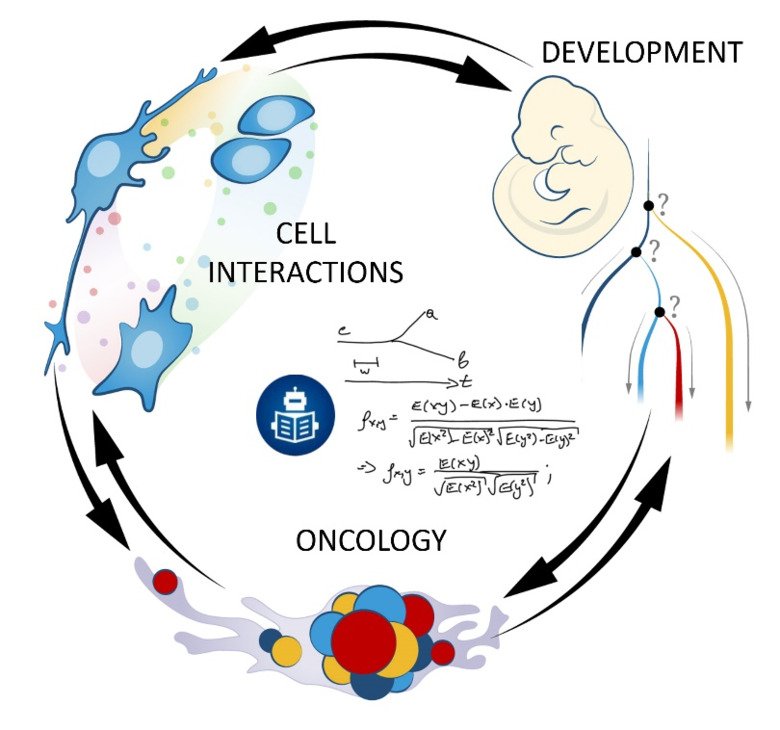
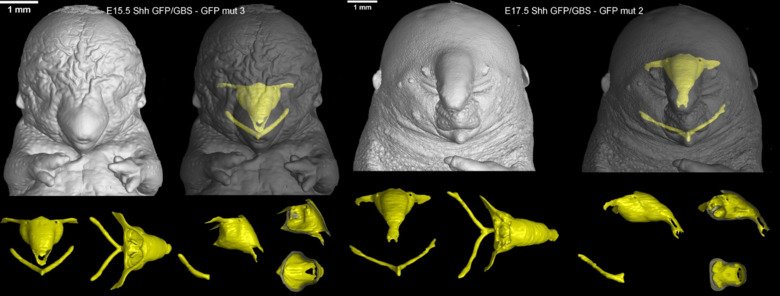
Our laboratory advances a broad spectrum of projects related to developmental biology, stem cells, EvoDevo and regenerative medicine. The methodology includes classical developmental biology approaches blended with single cell transcriptomics, 2D sequencing and 3D-reconstructions of tissues and organs based on optical or X-ray methods (micro-CT, synchrotron).
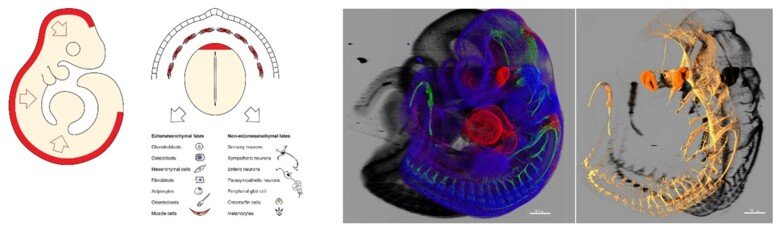
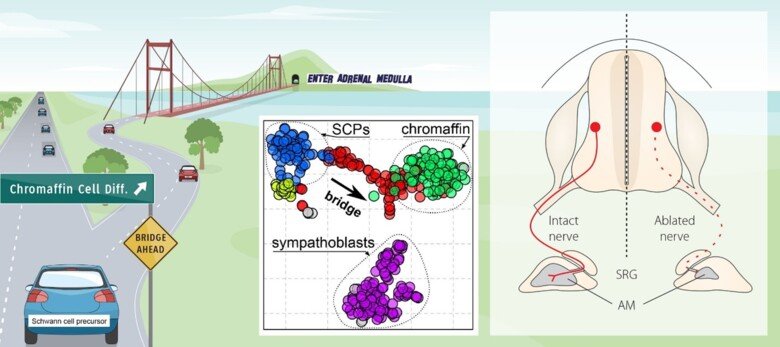
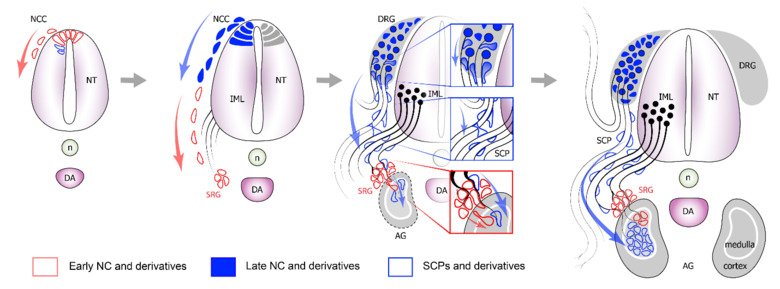
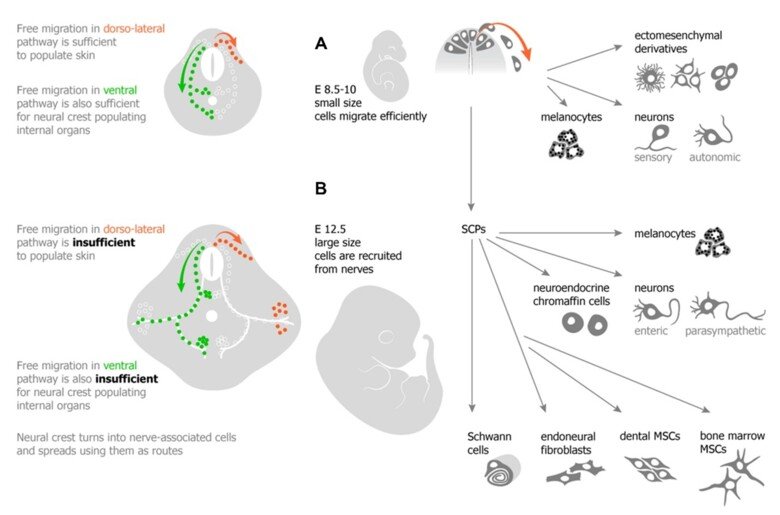
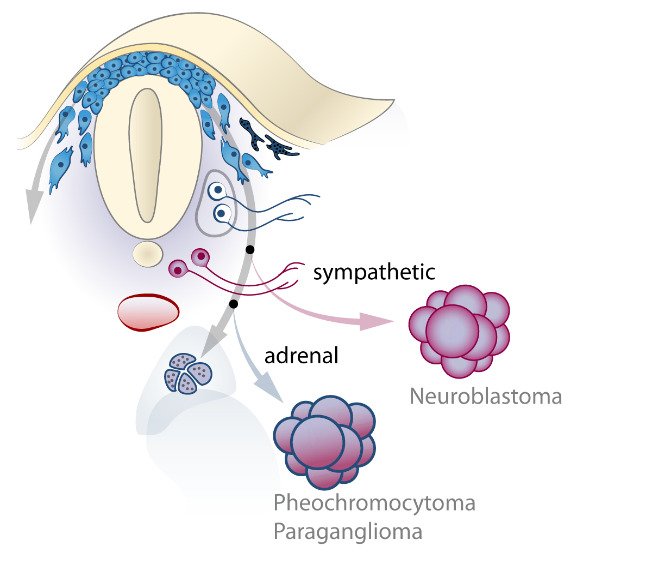
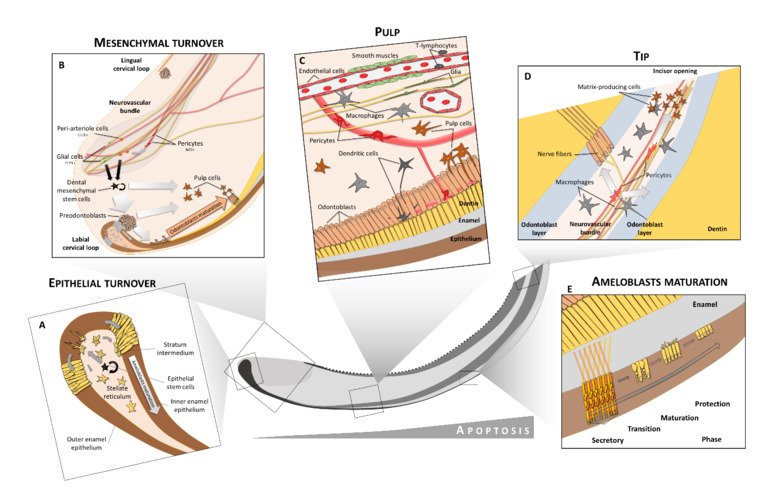
The neural crest stem cells is our primary model system, where we address general principles of cell fate choice, transcriptional and epigenetic control of a lineage progression, morphogenesis, and tissue shaping.
News from the group
 Photo: Creative Commons CC0
Photo: Creative Commons CC0Make a donation to our research
Your support means a lot to the success of research. This allows us to go further in our efforts to improve human health through research and education.