Research focus
Parkinson’s disease (PD) is the second most frequent neurodegenerative disorder and is diagnosed based on the appearance of tremor, rigidity, and bradykinesia, secondary to the loss of midbrain dopamine neurons projecting to the basal ganglia. In addition to the cardinal motor symptoms, PD is accompanied by a wide range of co-morbidities, which include olfactory and sleep disturbances, affective disorders, and cognitive impairment.
These conditions are often refractory to and may even be exacerbated by standard dopamine replacement therapies against PD. In addition, administration of L-DOPA or dopamine receptor agonists is often accompanied by the development of motor complications, or dyskinesia, and obsessive-compulsive disorders, including pathological gambling and addictive-like behavior.
Our work is based on the characterization of animal models reproducing these various disorders, combined with multiscale analyses to identify molecular, cellular and circuit abnormalities as potential targets for future treatments.
Non-motor symptoms in Parkinson’s disease
Our major research lines centers on the non-motor symptoms commonly observed in PD patients, which often appear during the prodromal phase of the disease. We have set up a toxin-based mouse model of early-stage PD, which reproduces several of these conditions.
We are currently using this model to investigate signaling and circuit abnormalities linked to anxiety and depression. Part of these studies have been carried out within the AND-PD consortium, supported by the Horizon2020 EU program. For a general presentation of current rodent models of PD-related affective disorders check our recent review.
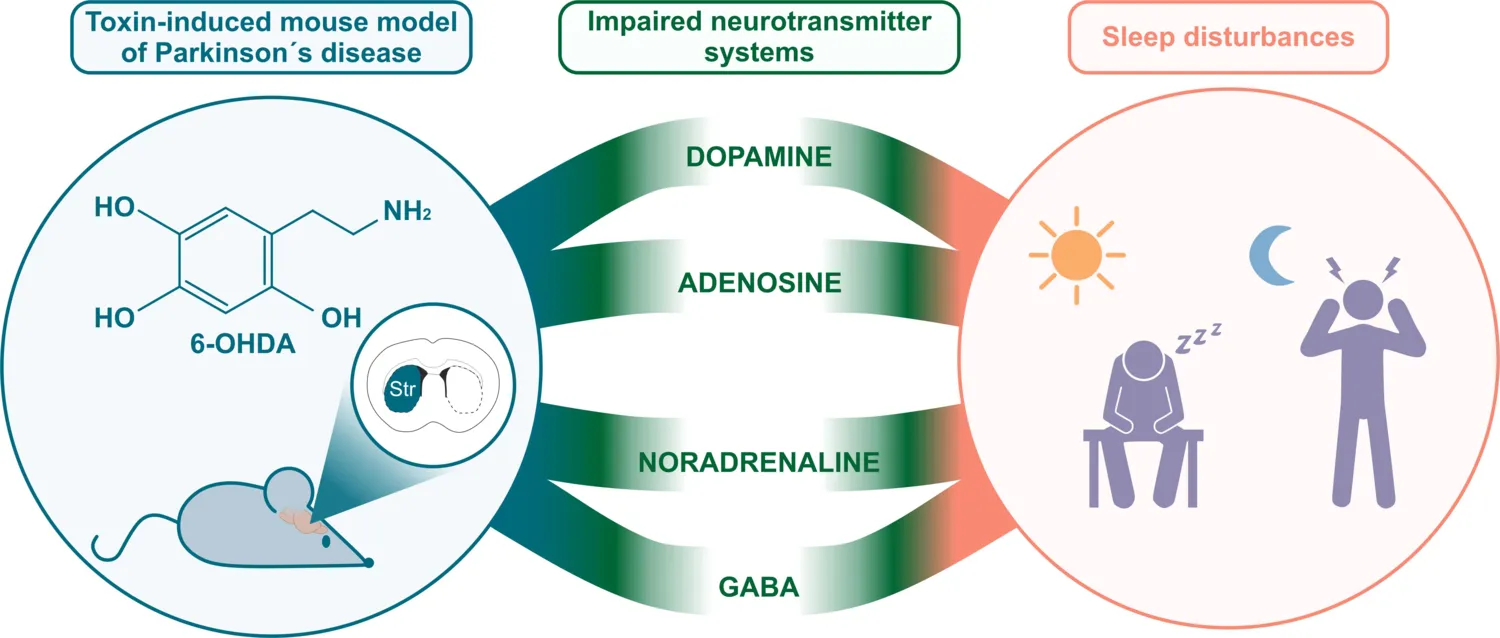
Other projects focus on circadian rhythm and sleep disorders, which are commonly observed in both de novo and medicated PD patients. Our mouse model of PD is characterized by disrupted rest/activity and endogenous circadian rhythm.
By applying polysomnographic recording to a similar experimental model, we have shown that these anomalies are accompanied by sleep disturbances commonly observed in PD patients, such as excessive day time sleepiness and sleep fragmentation.
Based on the potential translational value of this model, we are currently assessing the ability of specific pharmacological approaches to counteract sleep co-morbidities. As an example, in a recent collaboration with the pharmaceutical company, Umecrine Cognition, we are testing the possible effect of the neuro-steroid inhibitor, golexanolone, on PD-related excessive daytime sleepiness (see press release).
Molecular mechanisms of L-DOPA-induced dyskinesia
Our laboratory has identified several signal transduction components of the cAMP, ERK and mTOR cascades causally linked to the dystonic and choreic uncontrollable movements (dyskinesia) developed in response to administration of L-DOPA.
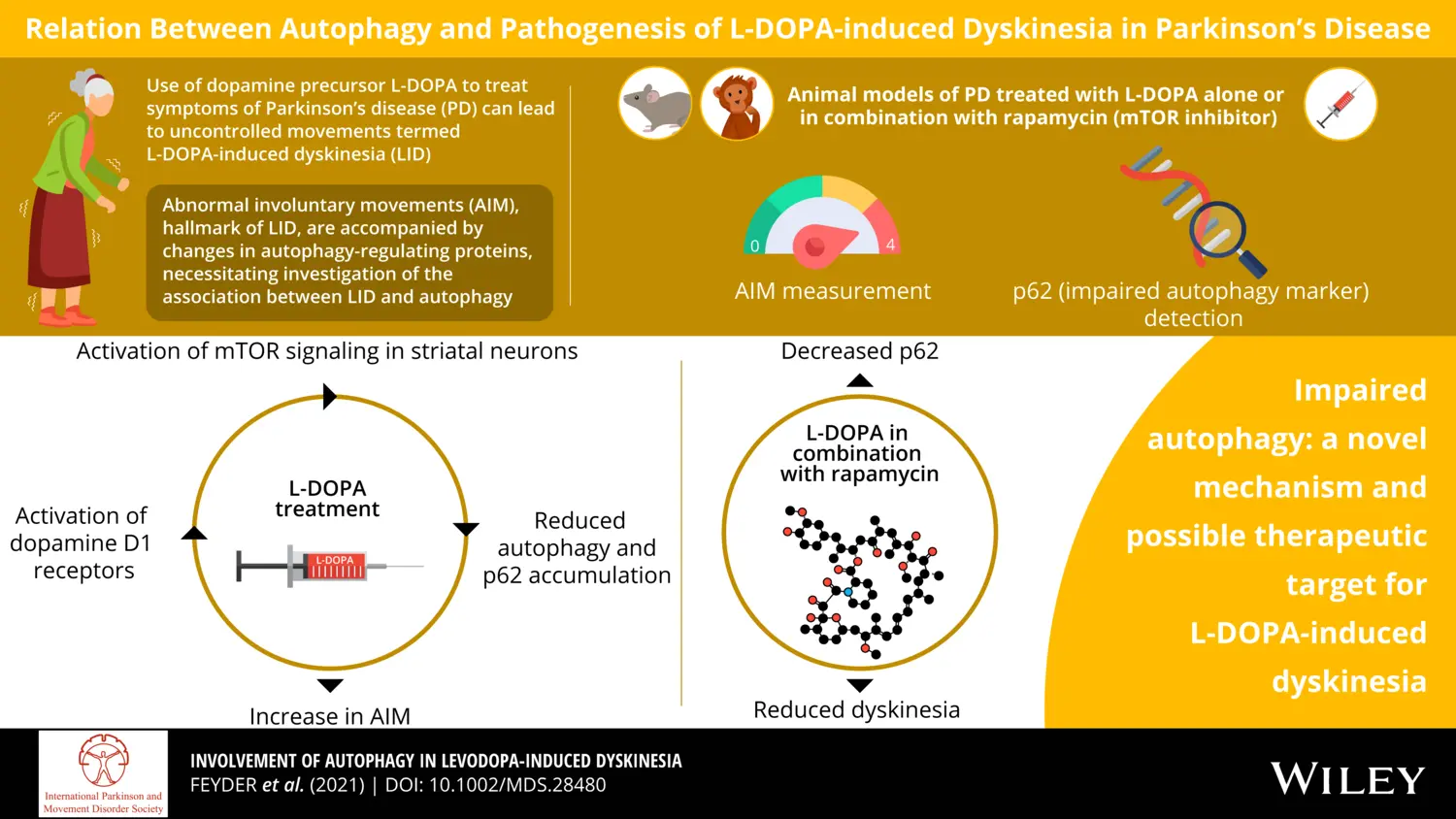
Recently, we provided evidence in support of the involvement of impaired autophagy in L-DOPA-induced dyskinesia.
This finding suggests that autophagy promoting drugs, including those approved for the treatment of cancer and metabolic disorders, may also be utilized as anti-dyskinetic agents.
Listen to the MD Podcast
Obsessive compulsive disorders in Parkinson’s disease
We are optimizing a mouse model of PD reproducing obsessive-compulsive disorders associated with the administration of dopamine replacement therapy. In a recent study, we focused on the addictive-like properties developed by L-DOPA and commonly referred to as dopamine-dysregulation syndrome. We found that this psychiatric complication is caused by the abnormal action of L-DOPA on sensitized dopamine D1 receptors.
Rewarding properties of L-Dopa in experimental parkinsonism are mediated by sensitized dopamine D1 receptors in the dorsal striatum.
Plewnia C, Masini D, Fisone G
Mol Psychiatry 2025 Mar;30(3):976-985