Our research
We use molecular, cellular and in vivo methods to study protein aggregation and defense mechanisms against it. Our work has unraveled molecular basis for aggregation of lung surfactant proteins, spider silk proteins and proteins associated with neurodegenerative diseases like Alzheimer and Parkinson. This has led to development of a drug against respiratory disease and establishment of new research groups at several universities.
BRICHOS as a treatment of Alzheimer's disease
We have shown that the chaperone-like domain BRICHOS passes the blood-brain barrier (BBB), prevents and treats amyloid-β (Aβ) toxicity related to Alzheimer, and described its molecular mechanism at atomic resolution, see Figure 1.
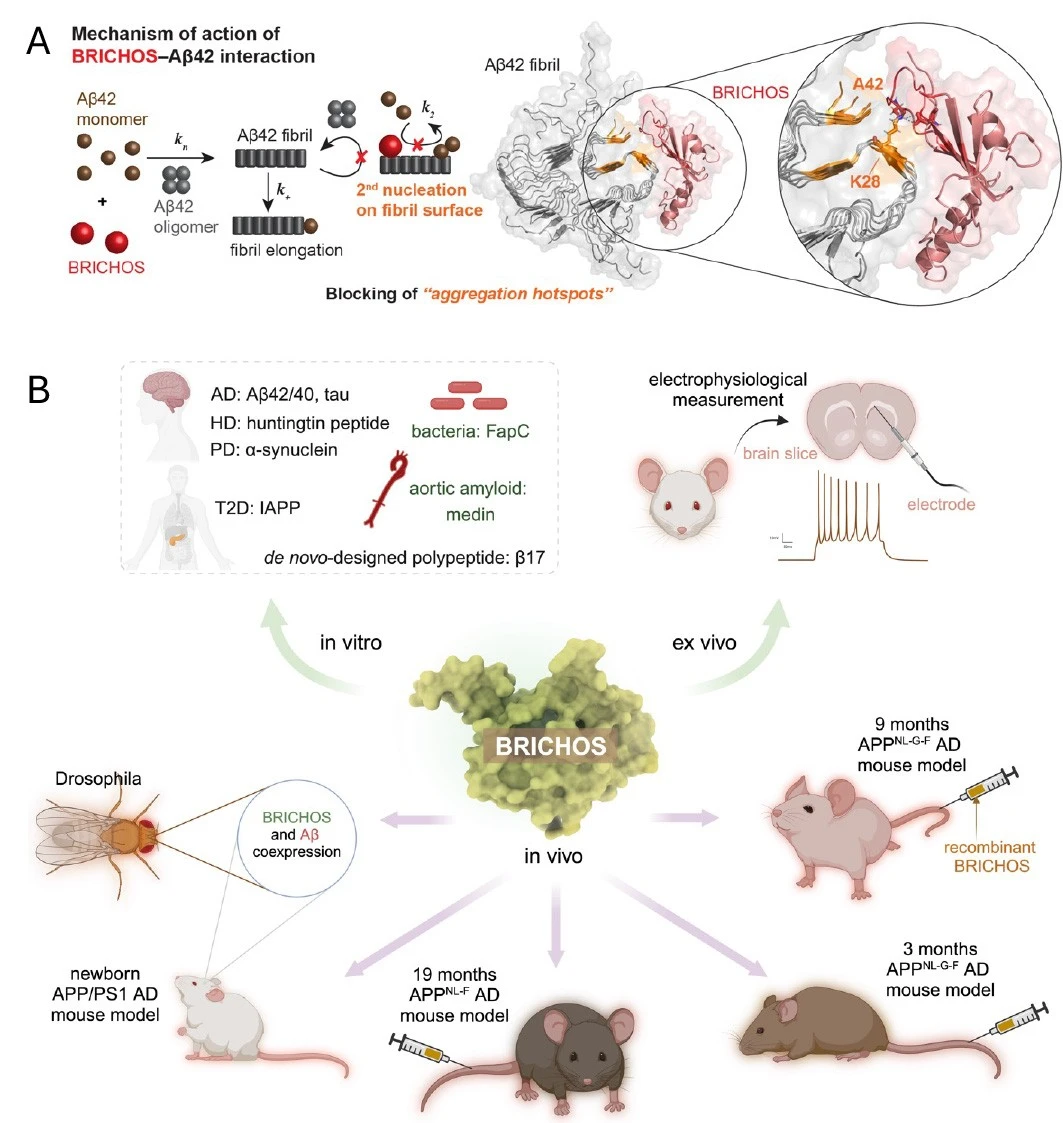
The properties of the BRICHOS make it very interesting as a possible treatment for Alzheimer's disease. We early found that survival and motor performance in Aβ producing fruit flies were improved by BRICHOS. Since monomeric Bri2 BRICHOS is most potent in preventing Aβ's neurotoxicity, we have designed a mutant (Bri2 BRICHOS R221E) that is more stable in its monomeric form. We have treated mouse models of Alzheimer's disease with intravenous injections of recombinant Bri2 BRICHOS R221E and demonstrated positive effects on cognition, amyloid plaques, neuroinflammation and on the expression of Alzheimer relevant genes.
These results are very promising for translation to a clinical situation and our focus now is to take BRICHOS as a treatment for Alzheimer's disease to clinical trials.
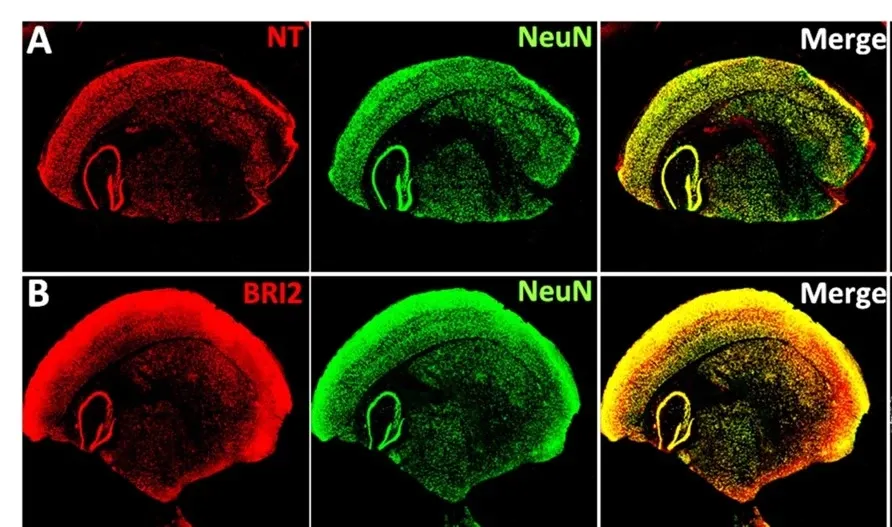
Blood brain barrier (BBB) passage
BRICHOS crosses the BBB in wildtype mice as well as in Alzheimer model mice. Moreover, we have found that cargo proteins fused to recombinant BRICHOS pass mouse BBB (Figure 2).
These results open the exciting possibility that BRICHOS can be used to transport various biologic drugs over the BBB. Our current aims in this project are to investigate the potency of BRICHOS to increase transport of biologic drugs against neurological diseases, for example anti-Aβ antibodies and lysosomal enzymes, over the BBB in various cell and animal models.
