Circadian rhythms
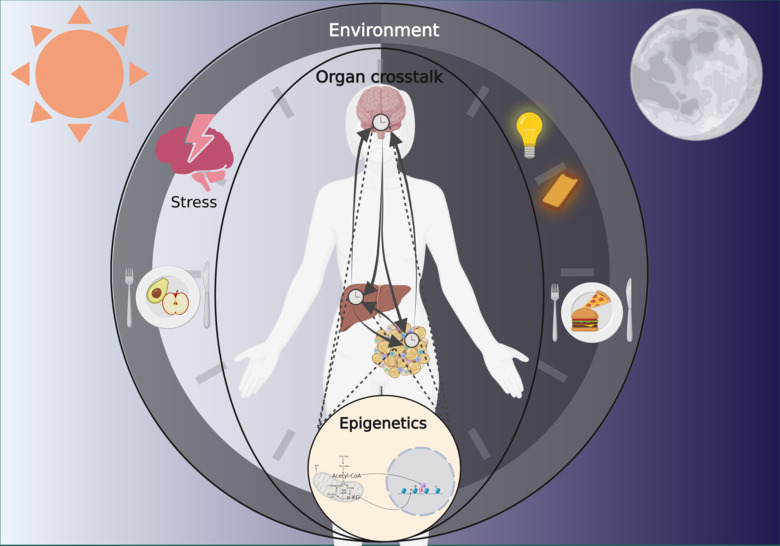
Like most other living organisms, humans have evolved to function in harmony with the day/night cycles. The biological processes following a ̴24-hour cycle are termed circadian (from the latin words Circa=about and Diem=day). Circadian rhythms are controlled by a genetically encoded “molecular clock” that exists in every nucleated cell and thus, in every organ. At its core, the molecular clock consists of a transcriptional-translational feedback loop regulating the rhythmic expression of thousands of genes. The clock in different tissues forms a network that communicates to align bodily functions with the time of day. Circadian disruption is caused by untimely light exposure, food intake and stress. Modern societies exist with artificial light at night, 24-hour access to hypercaloric foods and chronic stress.
Inter-organ communication
Our research focuses on understanding how environmental factors are integrated by the circadian system in specific organs and subsequently influencing rhythms in distant tissues. Circadian inter-organ communication involves several processes including metabolic signaling. The central clock is the main driver of systemic metabolic rhythms by controlling appetite and energy expenditure while the clocks in metabolically active organs, such as the liver and fat tissue, contribute to regulate metabolic rhythms by integrating nutritional cues.
Metabolic-epigenetic crosstalk
The circadian clock is tightly coupled with metabolism, as specific metabolites act as substrates and co-substrates for epigenetic regulators; factors changing the DNA structure without changing the sequence. Hence, circadian metabolic signaling from one organ can control rhythmic gene expression in a distant tissue. Thus, different tissue clocks cooperate to align behavioral and physiological rhythms to the time of day. Our project aims to delineate these mechanisms and how they relate to metabolic and mental health.
An overview of the research program