Our research
We are currently focusing on pancreatic beta-cell regeneration. Increasing the number of insulin-producing beta-cells might prove a better treatment for diabetes, which is at present controlled but not cured by insulin injections. Diabetes is characterized by elevated blood glucose levels, a consequence of insufficient insulin supply and/or insulin resistance. Despite mechanistic differences, both type 1 and late-stage type 2 diabetes feature depletion of beta-cells. Experimental ablation of beta-cells by chemical treatment or partial pancreatectomy in zebrafish and rodents is followed by significant recovery of the beta-cell mass, indicating that the pancreas has the capacity to regenerate. This regenerative capacity could potentially be exploited therapeutically - if the underlying mechanisms were better understood.
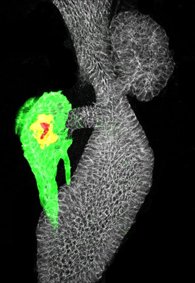
We perform unbiased chemical-genetic screens in zebrafish to identify compounds, signals and cellular mechanisms that promote beta-cell regeneration. The zebrafish model is particularly good for studying pancreatic development in vivo. First, the simplicity of its organ structures (e.g. the zebrafish embryo has only one pancreatic islet during the first week of development) allows rapid analysis of cellular changes. Second, zebrafish embryos are amenable to efficient transgenesis and drug delivery.
By using a wide range of techniques, we are investigating three different cellular mechanisms of beta-cell regeneration:
- Induction of beta-cell neogenesis
- Promotion of beta-cell proliferation
- Generation of ectopic insulin-producing cells
In sum, we aim to identify and characterize compounds, signalling pathways and cellular mechanisms that can induce or increase beta-cell regeneration, with the overarching goal of developing new therapies for diabetes.
