Research focus
Autism spectrum disorder (ASD) is an early onset neurodevelopmental condition characterized by challenges in social communication and interaction, together with restricted and repetitive behaviors and atypical sensory processing. Many individuals with ASD also experience gastrointestinal and immune dysfunction, sleep problems, epilepsy and anxiety. ASD is a complex, heterogeneous condition with a strong genetic basis. Environmental exposures during pregnancy and early life, such as maternal stress, infections, diet, antibiotics and immune activation, also influence neurodevelopmental trajectories. Understanding how genetic susceptibility interacts with early environmental factors is essential for clarifying why some children follow typical developmental trajectories while others develop neurodevelopmental profiles characteristic of ASD.
Increasing evidence points to the gut microbiota as one biological pathway through which genetic and environmental influences may converge. Microbial metabolites and structural components, including peptidoglycans, modulate immune signaling, microglial maturation, synaptic development, myelination and other processes central to neurodevelopment. These findings suggest that early microbial life may contribute to the variability observed in ASD and may influence developmental trajectories in genetically susceptible individuals.
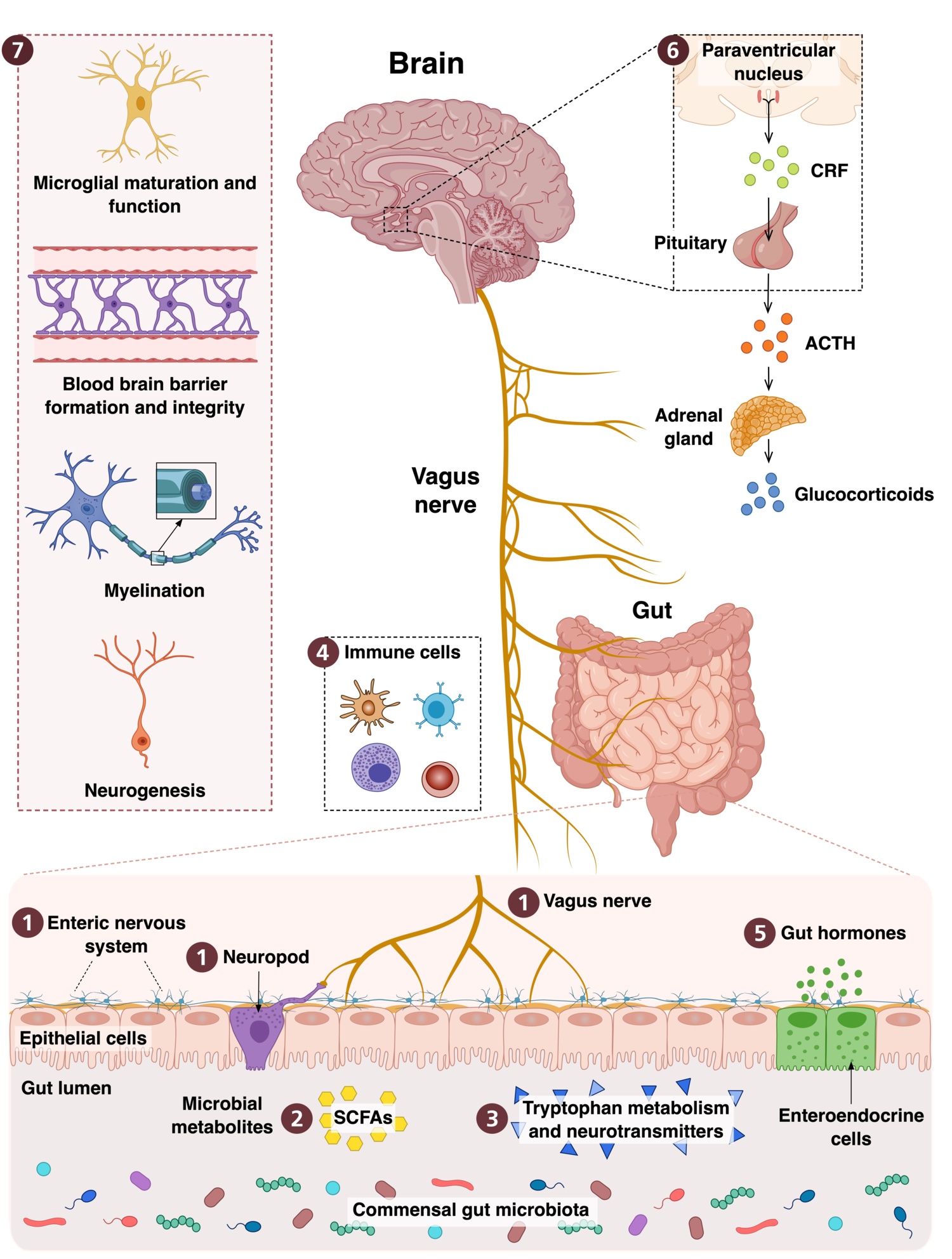
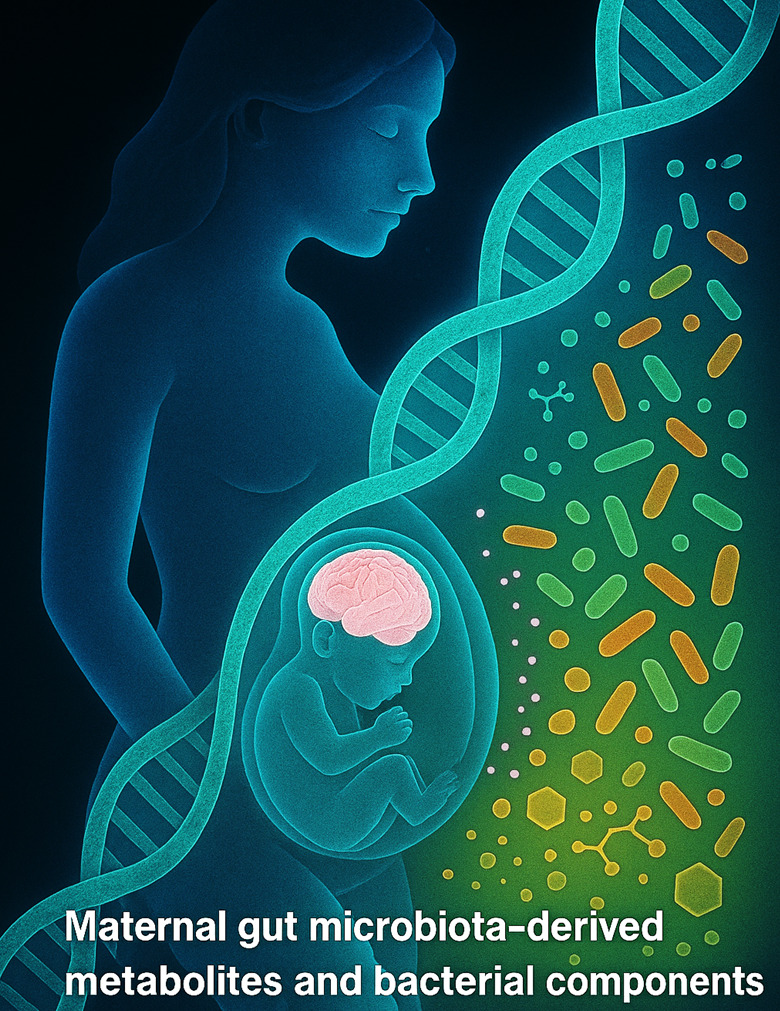
Our research investigates how the gut microbiota and its bioactive molecules shape brain development, brain function and behavior from the fetal period into adulthood. We focus on gut microbiota derived metabolites and peptidoglycans, and how these signals interact with genetic vulnerability and environmental exposures to influence ASD related neurodevelopmental outcomes. We use an integrative approach that includes animal models, human longitudinal cohorts and human derived tissues. We work closely with national and international collaborators, including clinicians, psychologists, microbiologists and immunologists, which strengthens our translational approach and supports continuous integration of basic, preclinical and clinical research.
Conceptual overview of Diaz Heijtz group research
Genetic susceptibility, environmental exposures, and early-life microbiota-derived signals influence fetal brain development and contribute to variation in neurodevelopmental trajectories.
Central Hypothesis
We propose that microbiota-derived factors (MDFs) play an essential role in shaping typical brain development. Disruptions in the levels or timing of these signals during sensitive developmental periods may perturb fundamental neurodevelopmental processes and increase susceptibility to neurodevelopmental conditions such as ASD. These effects may interact with genetic variation in pathways relevant to neurodevelopment and in microbial-sensing mechanisms, thereby amplifying vulnerability through gene–environment interactions within the microbiota–gut–brain axis.
Translational research approach
The research program integrates infant cohorts, gut microbiome and metabolome profiling, stem cell models and animal models, to investigate how microbiota derived signals influence typical and atypical brain development.
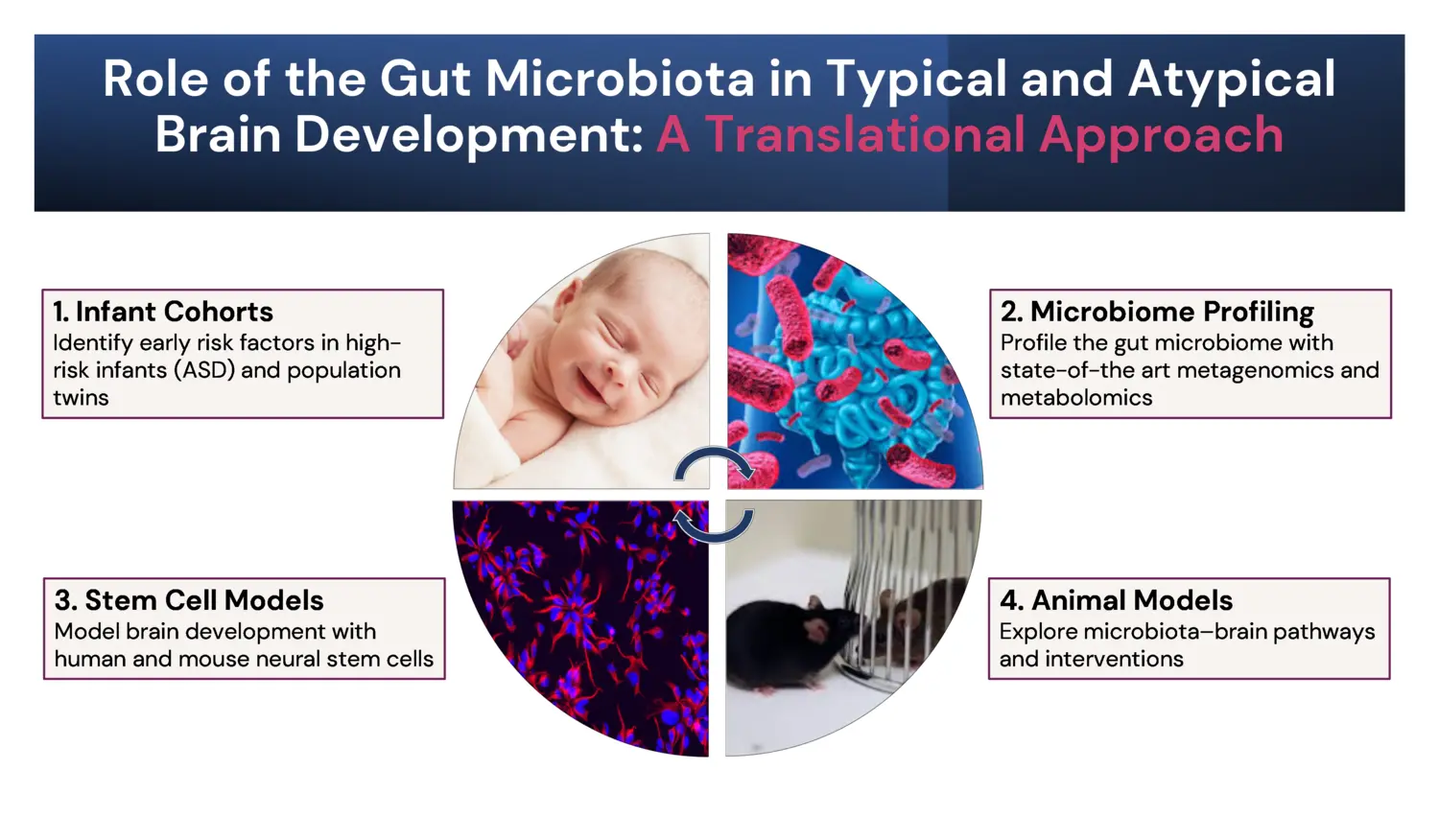
Recent Scientific Discoveries
Early differences in gut microbiota and metabolities in infants at elevated likelihood of ASD
In a prospective longitudinal study in infants at elevated likelihood of ASD (siblings of autistic children), published in Translational Psychiatry in 2023 (DOI: 10.1038/s41398-023-02556-6), we showed that already at five months these infants display distinct gut microbiota and metabolic profiles compared with infants without a family history of ASD. Elevated likelihood infants had reduced levels of Bifidobacterium species, key early colonizers important for folate synthesis, immune maturation and pathogen resistance, and increased levels of several Clostridium species, pathobionts associated with inflammation and ASD.
Metabolomic profiling revealed lower fecal GABA concentrations in the elevated likelihood group, which aligned with the distinct microbial composition. Importantly, these microbiota and metabolite differences appeared before any detectable behavioral alterations at 36 months of age and prior to differences in diet. Together, these findings highlight close relationships between early gut microbial ecology, metabolite production and developing neurobehavioral trajectories in infants with heightened genetic susceptibility to ASD.
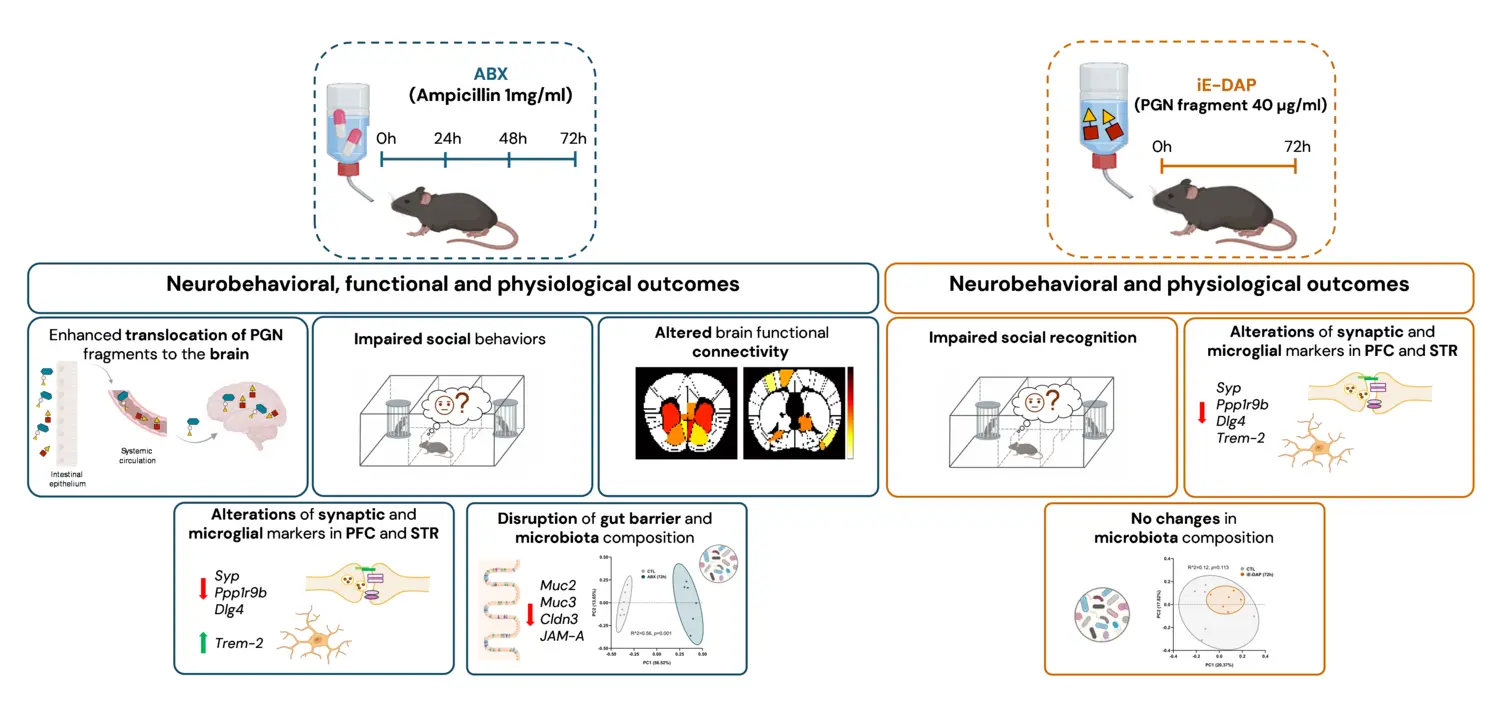
Short term antibiotic exposure promotes peptidoglycan activity in the brain and disrupts social behavior
Beta lactam antibiotics, among the most commonly prescribed medications, are known to disrupt the commensal gut microbiota and reduce microbial diversity. In a recent experimental study in mice, we demonstrated that short term exposure to ampicillin enhances the translocation of peptidoglycan fragments from the gut to the brain, establishing a novel mechanistic link between antibiotic induced microbiota disruption and central nervous system function. This translocation correlated with impaired social behavior, altered expression of synaptic and microglia related genes, reductions in functional brain connectivity, and changes in gut barrier integrity and microbial composition.
Peptidoglycan fragments from Gram negative bacteria replicated several behavioral and molecular effects observed with antibiotic treatment, further implicating peptidoglycan signaling pathways in the central nervous system effects of beta lactam antibiotics. These findings highlight potential risks of repeated antibiotic use for brain health, particularly in individuals genetically predisposed to neurological conditions.
Key research areas
- Role of gut microbiota derived metabolites and peptidoglycans during pregnancy and early postnatal life in shaping brain development and neurodevelopmental trajectories
- Effects of early life antibiotics and other environmental exposures such as stress on gut microbiota, brain circuits and behavior
- Gene environment interactions influencing ASD related neurodevelopment
- Longitudinal human cohorts, including infants at elevated likelihood of ASD and population twins
- Microbiota based early life interventions aimed at promoting healthy neurodevelopment and resilience
Keywords
Microbiota gut brain axis, Neurodevelopmental disorders, Autism spectrum disorder, Peptidoglycans

