NMDA receptors subunits as targets for the treatment of Parkinson’s disease
Parkinson’s disease is characterized by severe motor disturbance which include slowness of movement, rigidity and tremor. Anxiety, depression and anhedonia are also common non-motor symptoms. In Parkinson’s disease, different neuronal populations degenerate progressively, particularly dopamine neurons in the substantia nigra. This neuronal loss leads to a dramatic reduction in the content of dopamine in the striatum, and a resultant imbalance in several neurotransmitter systems in the basal ganglia including glutamatergic neurotransmission. Our research investigates, in models of Parkinson’s disease, if the functions of the NMDA type of glutamate receptors, and their subunit composition, are altered in different neuronal populations of the basal ganglia. An important goal of our research is to determine if the GluN2 subunits of NMDA receptors can be proposed as targets for the treatment of Parkinson’s disease.
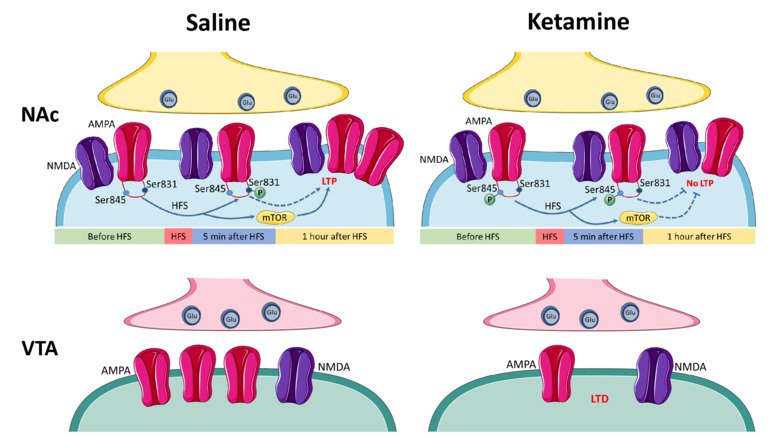
NMDA and AMPA receptors as targets for the treatment of depression
Low doses of ketamine exert rapid and lasting antidepressant actions in treatment-resistant patients affected by severe depression. Our research aims to better understand how ketamine produces a rapid and lasting antidepressant action and to suggest other therapies for the treatment of depression. We examine the molecular and cellular mechanisms by which ketamine and its metabolites alter glutamatergic transmission and plasticity in brain regions involved in reward-motivated behaviors and in depression. This mesolimbic circuit includes the nucleus accumbens and ventral tegmental area and is involved in reward and motivation, important functions which are impaired in patients with depression. Our studies will allow us to determine if the subunits that compose NMDA receptors and AMPA receptors can be suggested as targets for the development of novel pharmacological tools, with reduced propensity to induce side effects, in the treatment of depression.