Our research
The Bri2 BRICHOS protein – a key to treat neurodegenerative disease?
Chaperone activity and effect on Aβ aggregation
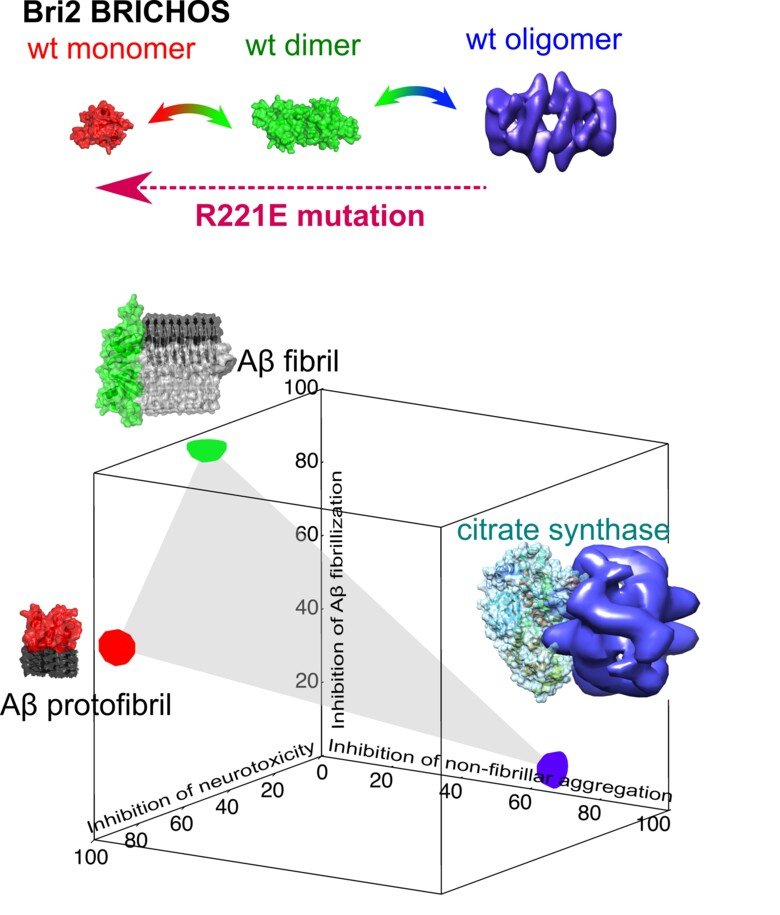
Finding ways to inhibit amyloid-associated toxicity is key to develop efficient therapeutics against amyloid diseases, where molecular chaperones are promising candidates. The chaperone domain BRICHOS is such an example that has been shown to specifically target and decrease toxicity associated with amyloid generation. BRICHOS from Bri2 is expressed in the brain and passes the blood-brain-barrier, and could hence be implemented in AD or PD treatments. Our group found that Bri2 BRICHOS exhibits different assembly states, which appear to execute most efficiently one distinct protective function, where monomers most efficiently suppress Aβ42-associated neurotoxicity and aggregation. Subsequently, we designed a Bri2 BRICHOS single point mutant that stabilizes the monomeric state. It selectively blocks secondary nucleation during Aβ42 aggregation and exhibits a significantly increased capacity to prevent Aβ42 toxicity to hippocampal network activity.
Effect on other amyloid systems
We investigate the effect of BRICHOS on other amyloid systems such as Parkinson’s related α-synuclein protein and Alzheimer’s associated Tau protein. Interestingly, we found that Bri2 BRICHOS exhibits an inhibitory effect on the aggregation on α-synuclein aggregation, where BRICHOS predominately inhibits surface-catalyzed secondary nucleation of α-synuclein. Furthermore, it binds to α-synuclein oligomers and suppresses α-synuclein-associated toxic effects. We currently study effects of molecular chaperones on liquid-liquid phase separation and droplet formation of amyloidogenic proteins and the link to amyloid formation and associated toxicity.
Identification of aggregation hotspots on the fibril surface
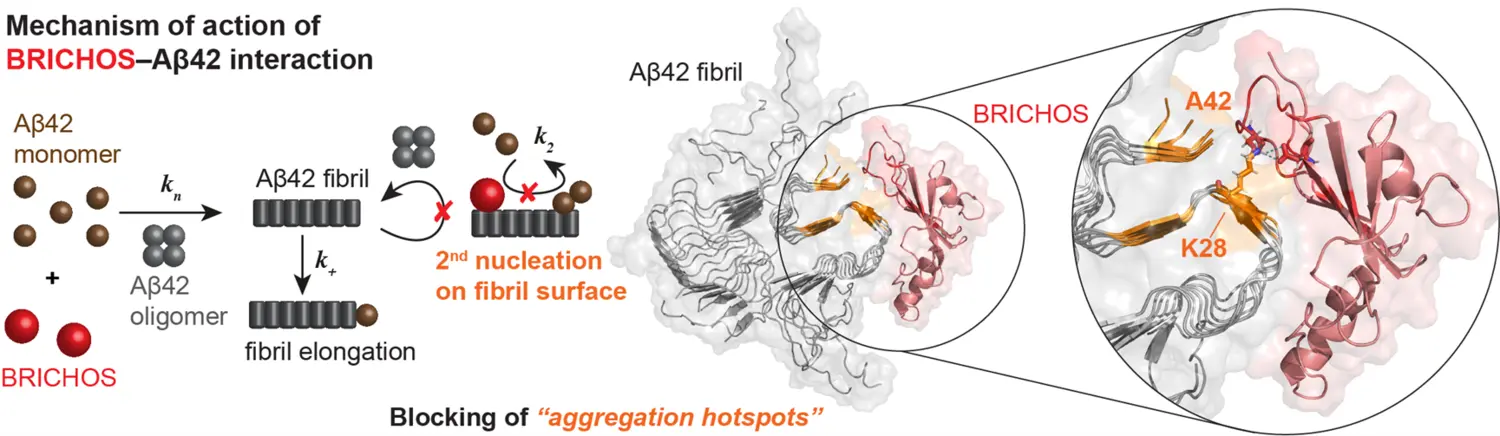
Utilizing the ability of BRICHOS to senses sites on amyloid fibrils where secondary nucleation occurs, we apply a combined approach of electron microscopy and solid-state NMR to obtain detailed insights into the modulation of the structure. For Aβ42 we found that the three C-terminal β-strands of the Aβ42 fibril are targeted by BRICHOS, which are potential aggregation hotspots on the fibril surface for secondary nucleation. We currently translate this approach to investigate interactions with α-synuclein and Tau fibrils.
Effect of metal ions and other aggregation modulators on amyloid-β self-assembly
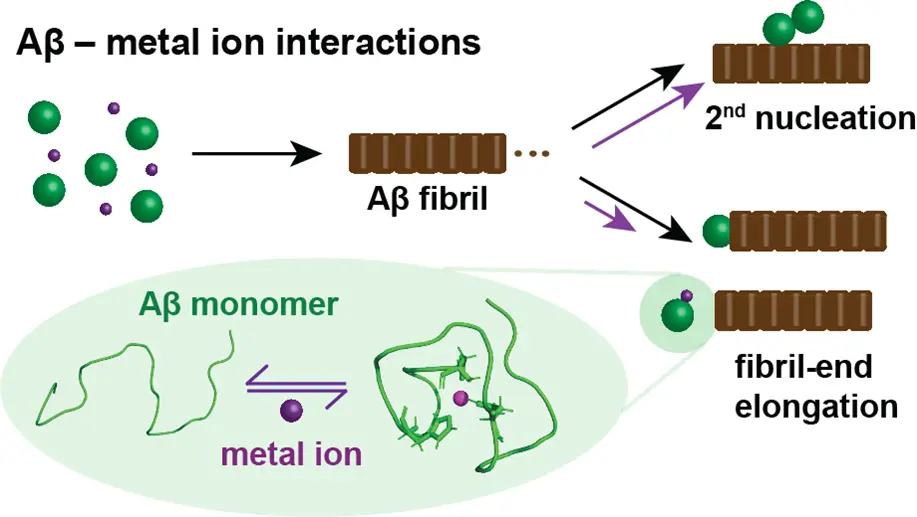
Metal ions are suggested to play a crucial role as aggregation modulators of Aβ aggregation, which is implicated in Alzheimer’s disease. Our group reported that copper, zinc and silver ions efficiently inhibit Aβ aggregation by retarding fibril elongation. Further, these metal ions bind monomeric Aβ, and form dynamic metal-ion bound complexes. Our studies also comprises other aggregation modulators to obtain detailed insights into the self-assembly mechanisms to provide the basis for drug development.
Molecular structure and neurotoxicity of in vivo-derived amyloid-β fibrils
Our research also concerns investigations of Aβ fibril structures derived from AD mouse models, since knowledge about molecular fibril structures and translation to the in vivo situation are the basis for design of novel amyloid inhibitors. In particular interactions with molecular chaperones are in our current research focus, where we apply high-resolution structural techniques.
Development of novel protein production protocols enabled by a customized spider silk domain
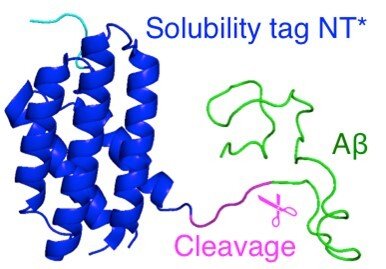
Another line of research is the development of facile systems for recombinant production amyloidogenic proteins and peptides. For Aβ, we have recently reported a protocol, which gives exceptionally high yields for monomers of different human Aβ variants based on a designed spider silk domain. This protocol facilitates production of aggregation-prone proteins, also in minimal medium, providing efficient and low-cost production of isotope-labeled proteins and peptides.
Development of novel protein-based biomaterials
Besides their association with diseases, amyloidogenic proteins are widely used in nature as building blocks of functional materials, which exhibit several outstanding properties. Our research aims to design and create new biomaterials based on spider silk proteins in combination with amyloidogenic proteins. In particular, the development of strong and specific metal ion-binding biomaterials is one research focus in our laboratory. Further, we develop methods to functionalize amyloid-based fibrils using the BRICHOS domain.
Work opportunities for Bachelor/Master, PhD and Post-doc students
If you are looking for a Bachelor & Master, PhD and Post-doc project and have the relevant background and interest in our research, please feel free to contact us for potential projects and vacancies.
