We are currently recruiting new team members! If you want to drive exciting projects as Postdoc, please contact: Lars Jakobsson via email: Lars.jakobsson@ki.se. Positions are fully funded.
Vascular morphogenesis and function in health and disease
Overview
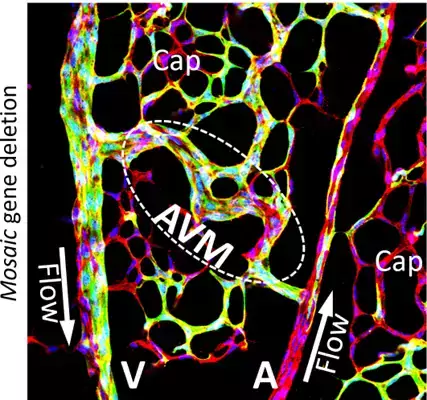
Dysfunction and mispatterning of the blood and lymphatic vasculatures are central in the progression or development of several diseases such as cancer, lymphedema, diabetic retinopathy, trauma, stroke complications, hereditary haemorrhagic telangiectasia (HHT) and ischemic heart disease.
The formation and function of the vascular system of arteries, capillaries and veins rely on properties of the components of the vascular wall; the endothelial cells (ECs), the supportive mural cells (pericytes and smooth muscle cells), astrocytes (in the CNS) and their shared basement membrane (BM).
Aim
We study molecular and cellular processes whereby the blood and lymphatic vasculatures adapt their size, architecture, and function to optimally meet the changing demands from tissue. The aim is to utilize the acquired knowledge to develop means to prevent vascular malformation and malfunction in disease. The research field is directly relevant to a vast number of human pathologies, including vascular anomalies, heart disease, stroke, diabetes complications, cancer, lymphedema, and inflammatory diseases.
Technology and resources
- Mice with inducible and cell specific genetic gain- and loss- of function in combination with a repertoire of conditional and cell specific reporters allowing for lineage tracing, clonal analysis and cell sorting.
- Multi-photon intravital imaging
- Confocal live cell imaging
- Fluidics and in vitro experimentation
- Tissue-clearing techniques
- Light-sheet microscopy
- scRNA-sequencing
- Large scale proteomics
- Mouse embryonic stem cell models
- AAV-mediated gene modulation