KCC and Vecura
The Karolinska Cell Therapy Center (KCC) is a resource at Karolinska University Hospital that is closely linked to Karolinska Institutet (KI) both via the core facility function for researchers at KI and through common strategic initiatives and financial support covering integrated operations.
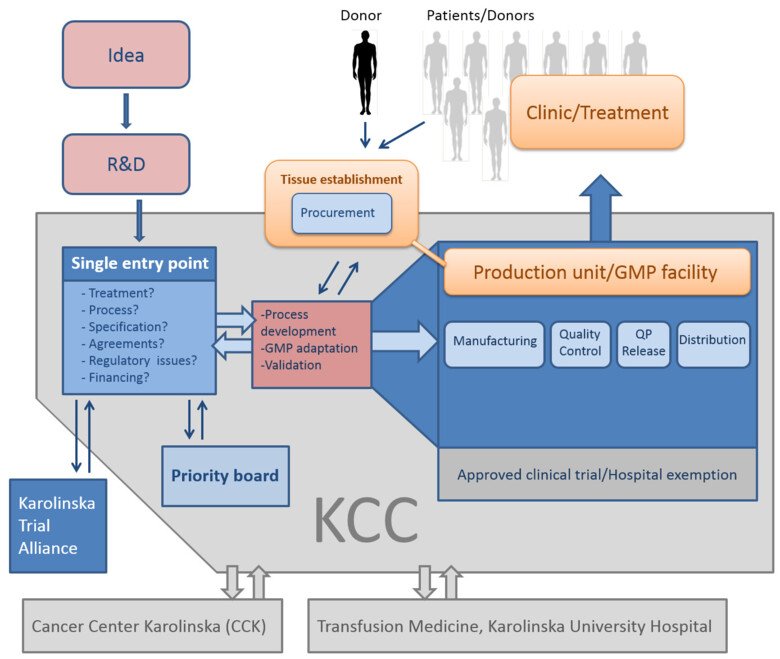
KCC supports and facilitates for researchers, clinicians and industrial companies that aim to develop quality assured Advanced Therapy Medicinal Products (ATMPs) and cell therapy products for unmet clinical needs. KCC is also organized to ensure patient safety and is the unit that administer all ATMP-operations at Karolinska University Hospital.
Single Entry Point
The Single Entry Point is a support function that guides the client through the required steps from research product to a quality assured product that can be used in clinical trials. Issues that may be discussed include regulatory issues, process development, process validation, treatment, agreements, financing etc.
Priority Board
The Priority Board interacts closely with the Single Entry Point and evaluates, approves and prioritizes projects within KCC and recommends who gets access to resources and support.
Production unit Vecura
The production unit Vecura is an integrated part of KCC. Other production units connected to KCC is the Cancer Center Karolinska (CCK) and Transfusion Medicine, Karolinska University Hospital. Vecura is situated in the Novum building at Campus Flemingsberg, and the unit offers expertise within regulatory issues, clean room production and quality analysis.
Competence and resources are available for developing customized production processes for the projects that have passed through the Single Entry Point and the Priority Board.
The facility is approved by the Swedish Medical Products Agency for the manufacturing of cell therapy products for clinical trials and have a tissue establishment permit from the Health and Social Care Inspectorate (IVO) for handling cells and tissues.
Contact
Karolinska University Hospital, Karolinska Cell Therapy Center
Acting Head of Unit: Pontus Blomberg
Visiting adress: Vecura, Novum, level 3, Hälsovägen 7, Huddinge
Postal adress: Karolinska Universitetssjukhuset Huddinge, Vecura, Novum, 141 86 Stockholm
E-mail: kcc.karolinska@sll.se
