Senior Grants 2023-2024
The aim of the SFO Stem cells and Regenerative medicine is to support research and infrastructure within the field of stem cells and regenerative medicine. The SFO supports researchers, research programs and infrastructure at the Karolinska Institutet (KI) with or without collaboration with the health care system based on scope, quality, and the potential to strengthen and develop the field.
The steering committee for SFO stem cells and regenerative medicine has decided to award 5 senior research grants for 2023 and 2024.
Each grant has a value of 2 million SEK per year.

Sten Eirik Jacobsen
Harnessing the power of T cell immunity to unravel the stem cell dependency of sustained normal and malignant hematopoiesis

No studies have addressed the dependency of normal steady-state adult hematopoiesis or hematological malignancies on stem cells, following efficient and selective elimination of the stem cells in vivo. We aim to resolve this issue in mouse models of normal and malignant hematopoiesis through engineering of T cell receptors (TCRs) efficiently and specifically targeting hematopoietic stem cells. While there are no means to address this optimally in humans, the findings in mouse models will have important implications for the human cancer stem cell hypothesis and therapies.
More research is performed in Sten Eirik Jacobsen group at Department of Medicine, Huddinge (MedH) and Department of Cell and Molecular Biology (CMB).

Sten Linnarsson
Targeting glioblastoma stem-like cells using engineered lipid nanoparticles
Glioblastoma is thought to arise due to transformation of residual stem cells (hierarchical model) or dedifferentiation of post-mitotic glia (stochastic model); either way, stem-like cells drive the development of tumors. However, despite decades of efforts to develop targeted therapies, overall survival has remained poor. We will develop DNA lipid nanoparticle vectors targeting diverse glioblastoma stem cell states. Lipid nanoparticles are promising for their flexibility of design, simple manufacturing and ability to target different cell types, exemplified by the successful development of mRNA-LNP Covid-19 vaccines.
Less work has focused on LNP formulations with DNA payload, which provide an opportunity for longer-term modulation of cellular function compared to mRNA-based LNPs, and for programmability based on regulatory DNA elements.
More research is performed in Sten Linnarsson group.

Gonçalo Castelo-Branco
Establishment of regenerative cell statesin multiple sclerosis: identifications of key transcription factors for oligodendrogenesis
Multiple sclerosis (MS) is characterized by an auto-immune attack on oligodendrocyte (OL)-derived myelin. Spontaneous remyelination driven by oligodendrocyte precursor (OPCs) and mature OLs has been described in MS, but eventually fails. We will determine how different OL-lineage cell states are established and identify transcription factors underlying their establishment.
We will perform pool CRISPR-based screenings, combining with single-cell and spatial omics, and assess the effect of these transcription factors on the differentiation/myelination potential of human OLs. This project will unveil new strategies to drive OL lineage to remyelinating states, which might lead to regenerative medicine approaches for MS.
More research is performed in Gonçalo Castelo-Branco group.

Maria Eriksson
Vascular regeneration and functional implications from somatic mutations in age-associated vascular disease
We and others have analyzed somatic mutations in human tissues and showed that somatic cells accumulate thousands of mutations during development and aging.
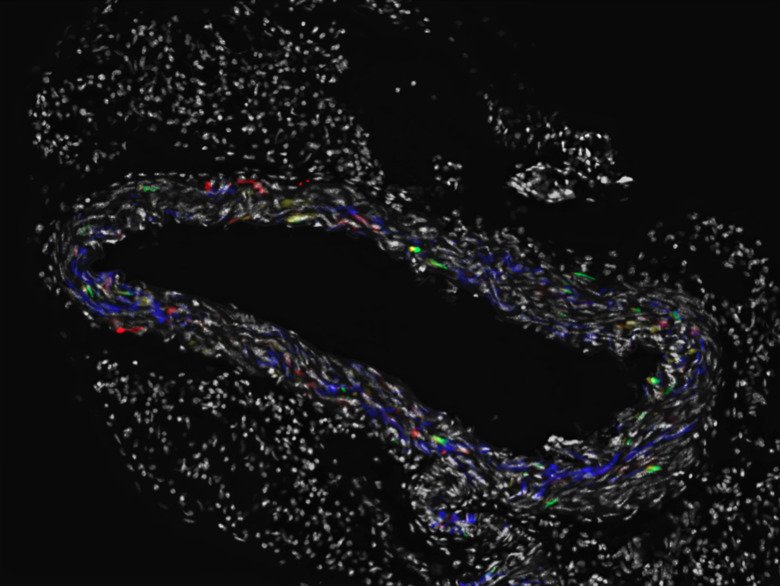
While most of the mutations are likely harmless, some either contribute to disease and aging or are directly disease-causative, as in the formation of tumors in cancer. In this study, we will develop models to study clonal expansion of somatic mutations in the arterial wall during aging and disease.
More research is performed in Maria Eriksson group.

Kirsty Spalding
Cell regeneration in the adult human kidney in health and disease
Chronic kidney disease is estimated to affect more than 10% of the population. With no curative treatments to restore kidney function, clinical efforts have instead focused on slowing the progressive decline into renal failure. The potential for stimulating the regeneration of podocytes – the key cells of the glomerular filtration barrier whose loss is directly correlated with a decline in function – is of immense clinical interest.
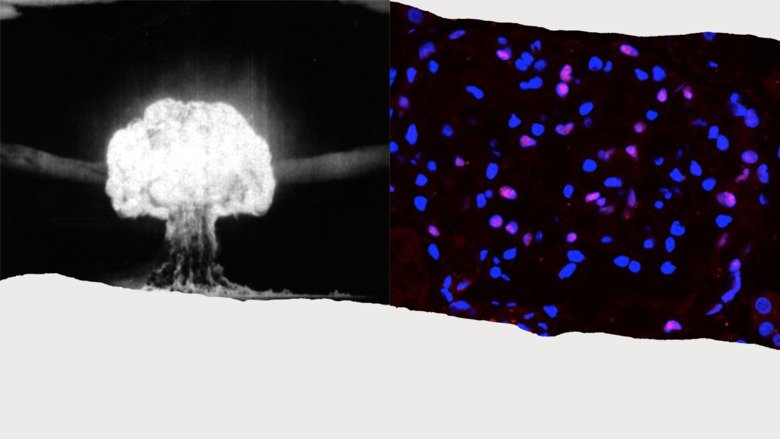
We will use carbon-14 dating of nuclear DNA to determine the regenerative capacity of podocytes in the adult human kidney and assess whether stimulating endogenous podocyte cell replacement is a viable therapeutic option for kidney disease.
More research is performed in Kirsty Spalding group.
