Junior Grants 2025-2026
The aim of the call is to support excellent research within the field of stem cells and regenerative medicine at Karolinska Institutet (KI). Research on the generation of cells, tissues and organs as well as mechanisms important for regeneration is important for understanding normal development, homeostasis, and physiological as well as pathological processes. Advancing our knowledge about these processes is critical within regenerative medicine and tissue engineering.
The steering group for SFO stem cells and regenerative medicine has decided to award 4 junior research grants for 2025 and 2026.
Each grant has a value of 2 million SEK per year.
Marco Gerling

Modulating Liver Regeneration to Restrain Tumor Growth
Tumor invasion into the liver triggers a regenerative response that can either promote invasion or encapsulate the tumor in a reparative capsule. In this project, we explore whether modulating this regenerative response can restrain tumor growth, using liver metastases models and clinical samples. With pharmacological and CRISPR-based approaches, we aim to amplify or suppress liver regeneration and assess its impact on tumor invasion. By integrating spatial imaging and single-cell analyses, we seek to uncover key tumor-liver interactions. In the long run, our findings could drive novel regeneration-based therapies against aggressive liver tumors.
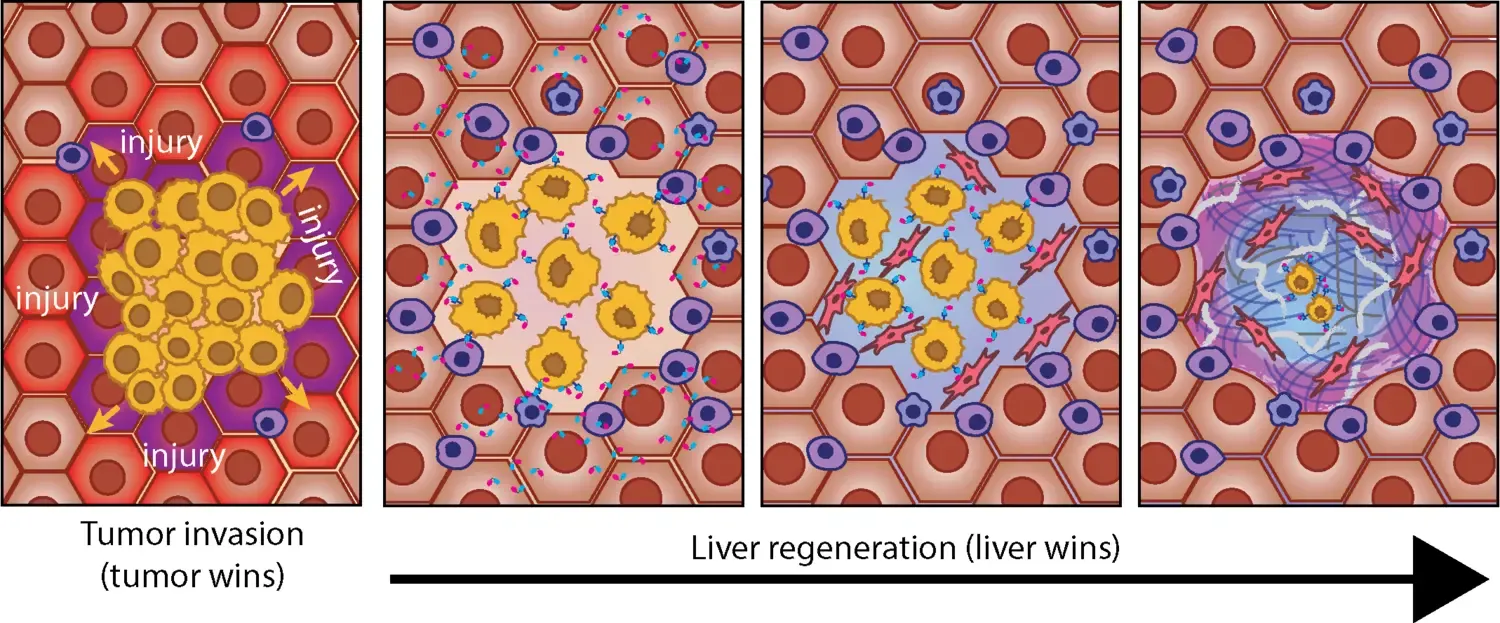
More research is performed in Marco Gerlings group.
Jeroen Goos

Click-and-trace: Precision tracking of therapeutic cells using bioorthogonal click chemistry
Cell-based therapies are demonstrating exceptional potential in the clinical setting. However, there is an immense gap in understanding what happens to the cells after their injection. In 60-80% of immunotherapy and 15-30% of stem cell therapy cases this leads to persistently ineffective treatments. To irrefutably determine the fate of the cells, my team is developing an innovative strategy to track the cells in the body using bioorthogonal click chemistry and molecular imaging.
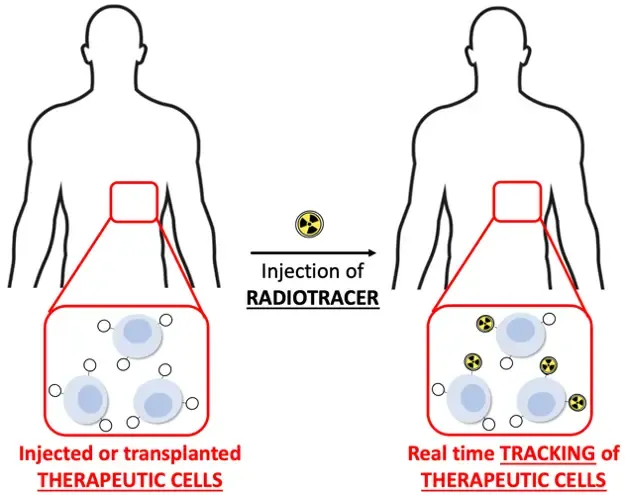
Our approach integrates recent advances in cell therapy, chemistry and nuclear medicine into novel cell tracking methodology. A better understanding of cell distribution in the body will improve the efficacy of cell-based therapies in general and aid in predicting therapy response and relapse.
More information on Geroen Goos KI page.
Jens Magnusson

Data-driven engineering of transcriptional networks for regenerative medicine via synthetic biology
Synthetic biology seeks to make biology easier to engineer by combining advances in genome engineering, computer algorithms, nucleotide synthesis and sequencing. This project will use synthetic biology to address two key challenges in regenerative medicine: improving the quality of stem cell differentiation using data-driven gene circuitry, and developing therapeutic cells that carry designer gene programs for regenerative functions.
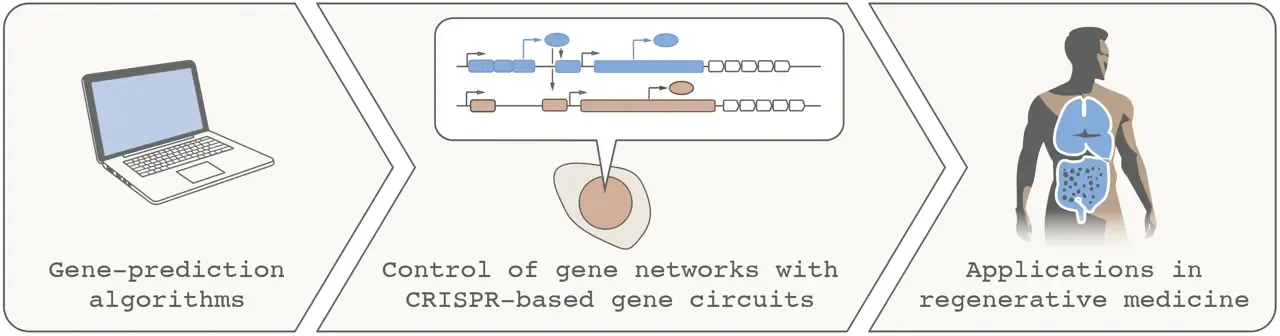
With the generous support of the StratRegen Junior Grant, we will aim to demonstrate how synthetic biology technology can be leveraged to program complex cell behaviors useful for tissue repair.
More information on Jens Magnusson KI page.
Michael Ratz

Scalable mapping of neural circuit assembly and dysfunction at molecular resolution
The mammalian brain contains many different neuron types which form neural circuits with remarkable precision. Single-cell RNA-seq is a powerful tool to catalogue the diversity of neuron types in the mammalian brain, but the molecular logic governing circuit assembly and maintenance remains obscure.
We will employ a novel approach based on barcoded circuit tracing and 3D intact-tissue RNA-seq for high-throughput mapping of synaptic networks. Comparative circuit tracing across different mouse models of autism spectrum disorder (ASD) will uncover the impact of mutations in ASD risk genes on neuronal cell identity and wiring.
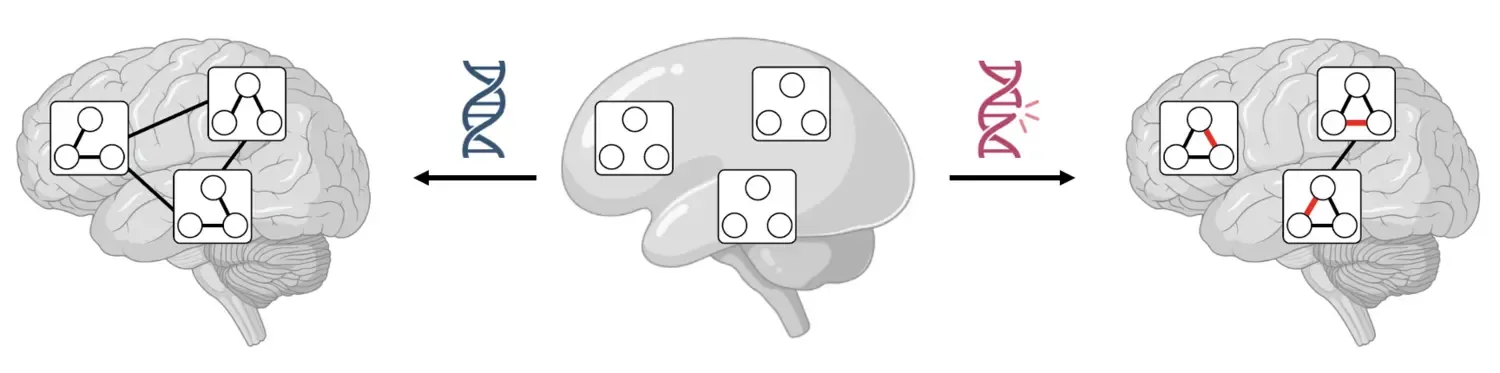
More research is performed in Michael Ratz group.
