Junior Grants 2023-2024
The aim of the SFO Stem cells and Regenerative medicine is to support research and infrastructure within the field of stem cells and regenerative medicine. The SFO supports researchers, research programs and infrastructure at the Karolinska Institutet (KI) with or without collaboration with the health care system based on scope, quality, and the potential to strengthen and develop the field.
The steering committee for SFO stem cells and regenerative medicine has decided to award 5 junior research grants for 2023 and 2024.
Each grant has a value of 2 million SEK per year.

Ulrika Marklund
Exploring the regenerating enteric nervous system for applications in restorative therapy of gastrointestinal disease
Neuropathy within the enteric nervous system (ENS) contribute to congenital, degenerative and inflammatory gut disorders that lack satisfactory treatment. Following injury or inflammation, regeneration of both enteric neurons and glia have been observed, yet without that functional deficits are restored.
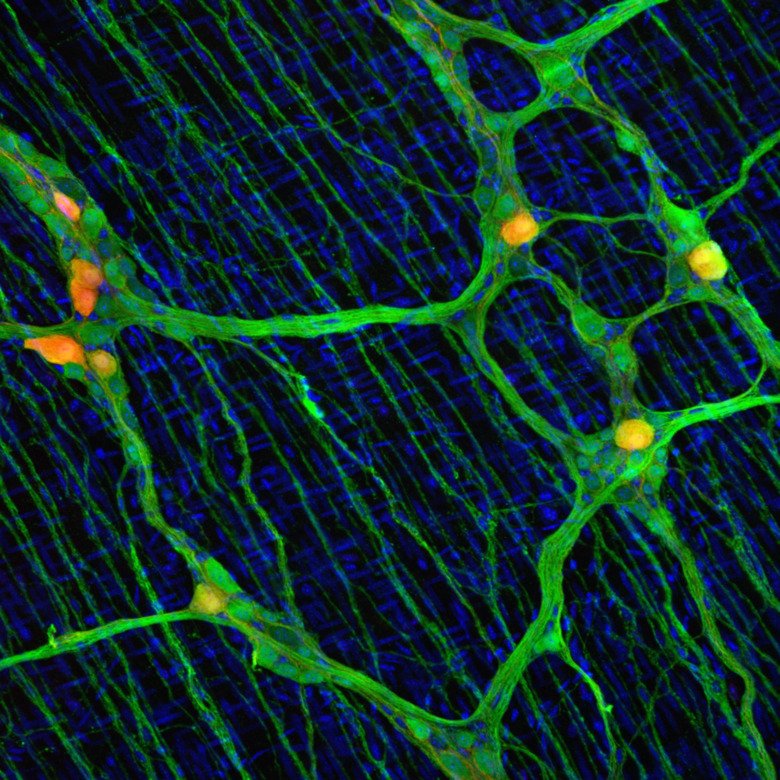
To achieve functional recovery, a balanced neuronal constitution would need to be re-created. We have recently determined general principles of embryonic diversification of enteric neuron subtypes, but the neuron identities emerging during adult neurogenesis remains unknown. In this project we aim to explore the potential and constraints of the regenerating ENS to pave the way for new regenerative strategies for treating neural deficits in the gut.
More research is performed in Ulrika Marklund group.

Simon Elsässer
Epigenetic mechanism of the first human embryonic lineage choices
The first lineage choice made in human embryo development separates trophectoderm (TE) from the inner cell mass (ICM), separating extra- and embryonic cell fates. Upon implantation, TE gives rise to placental tissues while the ICM progresses via the epiblast stage to form the fetus. Together with Fredik Lanner’s group, we have been studying how epiplast identity is specified and maintained in a human embryonic stem cell model.
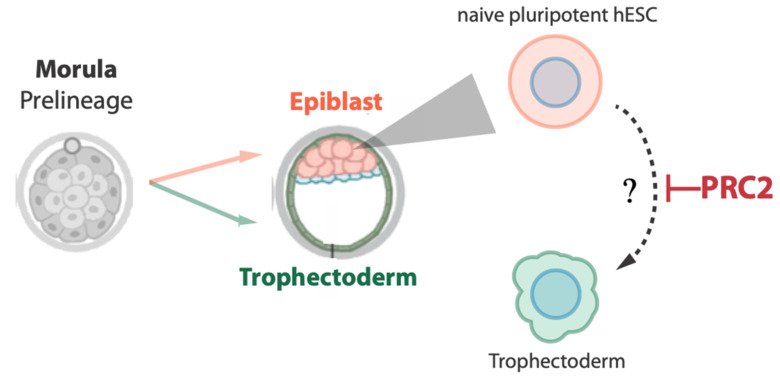
In the project, we will identify and study so-called epigenetic factors that guide cell fate decisions or provide barriers between different lineages in early human development, together orchestrating the ordered development of embryo and extraembryonic support tissues.
More research is performed in Simon Elsässer group.

Maria Genander
Regionalization of esophageal progenitor cell fate and tumor potential
In many stem cell niches, stem and progenitor cells are tucked away in anatomically distinct locations, enabling exclusive interactions with niche signals that maintain stemness and prevent differentiation. In structurally simple epithelia such as the esophagus, no local stem cell niches have yet been identified.
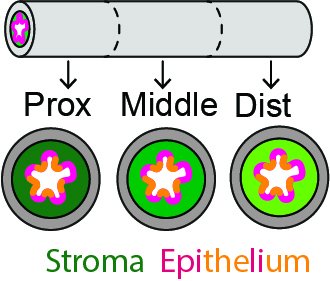
Our work suggest that symmetrically fated progenitor cells are enriched in specific epithelial regions, indicating that local signal environments, or niches, impact progenitor fate and subsequently susceptibility to transformation. Here, we propose to characterize how progenitor cell location impacts esophageal homeostasis and tumor initiation.
More research in performed in Maria Genander group.

Joel Nordin
Development of an in vivo gene editing technology of hematopoietic stem cells to treat X-linked agammaglobulinemia
This project aims to use gene-editing of hematopoietic stem cells (HSCs) to treat an inherited immunodeficiency, X-linked agammaglobulinemia (XLA), at Karolinska University Hospital. XLA patients carry a mutated BTK gene, thereby, lacking a functional BTK. Together with researchers at UCLA and close collaborators at KI, HSCs from XLA patients will be gene-edited by homology-directed, so-called CRISPR methods.
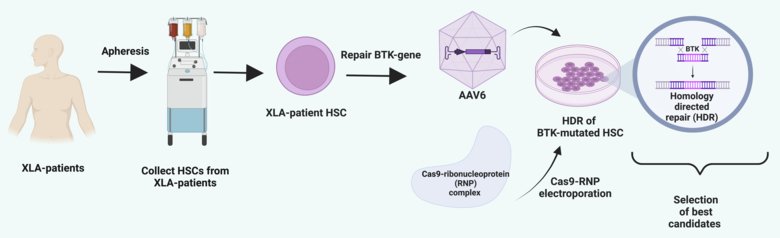
Hopefully, this preclinical attempt will restore the expression of normal BTK protein, which is non-functional in XLA patients, and pave the way for a clinical trial. In parallel, the project aims to increase the editing efficiency of HSCs by developing a novel delivery system of Cas9-RNPs (components of CRISPR) to enhance editing efficiency.
KI collaborators:
Molecular Cell Biology and Gene Therapy Science
Research group - Samir EL Andaloussi
More research is performed in Joel Nordin group.

Vanessa Lundin
Molecular pathogenesis of germline cancer predisposition in hematopoietic stem cells
Myeloid malignancies are clonal disorders of hematopoietic stem cells. We aim to better understand how certain genetic mutations in these cells predispose and lead to development of myeloid neoplasms, such as MDS and AML. In this project, we use patient-derived induced pluripotent stem cells (iPSC) from germline carriers of disease-prone variants. These personalized cell lines provide a unique opportunity to model and study the continuum of myeloid disease in vitro, as iPSCs can be readily differentiated into blood cells but also allow for genetic engineering, functional readouts, and validation of new drug targets.
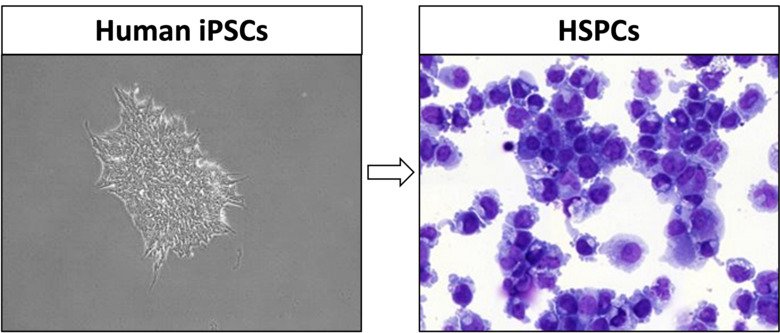
Leveraging state-of-the-art multiomics, we will obtain mechanistic insights into the pathogenesis of hematopoietic stem and progenitor cells (HSPCs) to ultimately identify strategies towards blocking malignant transformation and restoring hematopoietic output in myeloid malignancies.
More research is performed in Vanessa Lundin group.
