Senior Grants 2025-2026
The aim of the call is to support excellent research within the field of stem cells and regenerative medicine at Karolinska Institutet (KI). Research on the generation of cells, tissues and organs as well as mechanisms important for regeneration is important for understanding normal development, homeostasis, and physiological as well as pathological processes. Advancing our knowledge about these processes is critical within regenerative medicine and tissue engineering.
The steering committee for SFO stem cells and regenerative medicine has decided to award 6 senior research grants for 2025 and 2026.
Each grant has a value of 2 million SEK per year.
Emma R Andersson

In utero next generation single cell lineage tracing of ectoderm and mesoderm to reveal organ-specific mesenchymal lineages
We aim to develop a new method to lineage trace mesenchymal cells, which are essential for organ development, regeneration, and disease response. Current lineage-tracing methods for mesenchymal cells are confounded by hybrid cell identities. To overcome this, we will develop a Cre-independent, single-cell barcode-based tracing system to map mesenchymal origins in an unbiased manner, using in utero nano-injection.
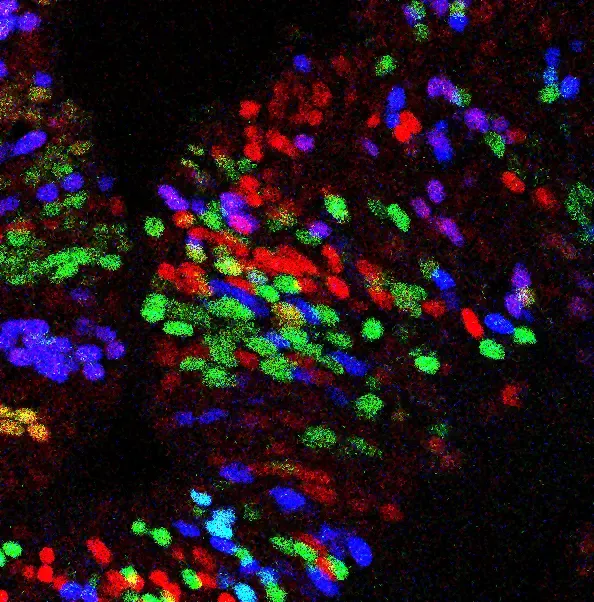
The method will be applied to resolving mesenchymal lineages and gene function in heart and liver development. The study integrates single-cell RNA sequencing and CRISPR-based perturbation, to unravel mesenchymal lineage contributions, with implications for regenerative medicine, fibrosis research, and organ repair strategies.
More research is performed in Emma Andersson´s group.
Pauliina Damdimopoulou

Regenerating human ovaries in vitro
Human fertility rates are declining globally, with 1 in 6 individuals experiencing involuntary infertility. While infertility treatments are widely accessible, a third of couples remain childless despite them. To better understand infertility, models that mimic human ovaries are needed—not only to uncover its causes but also to support ovarian follicle growth for oocyte production. In this project, we will leverage our extensive single-cell datasets on human ovaries to reconstruct the fertile somatic cell environment on biosilk in vitro.
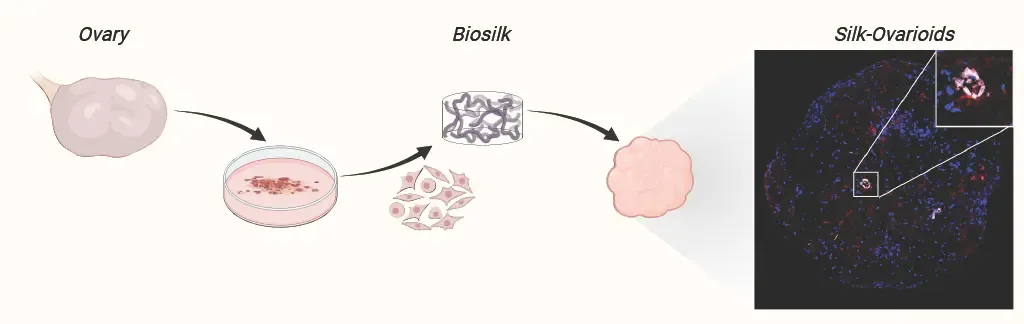
The ultimate goal is to develop biosilk-ovarioids as scaffolds for follicle growth—a tool with potential applications in reproductive toxicity testing, drug discovery, and even patient-specific oocyte generation.
More research is performed in Pauliina Damdimopoulou´s group.
Jonas Frisén

Analysis of cell lineages and turnover at single cell resolution in humans
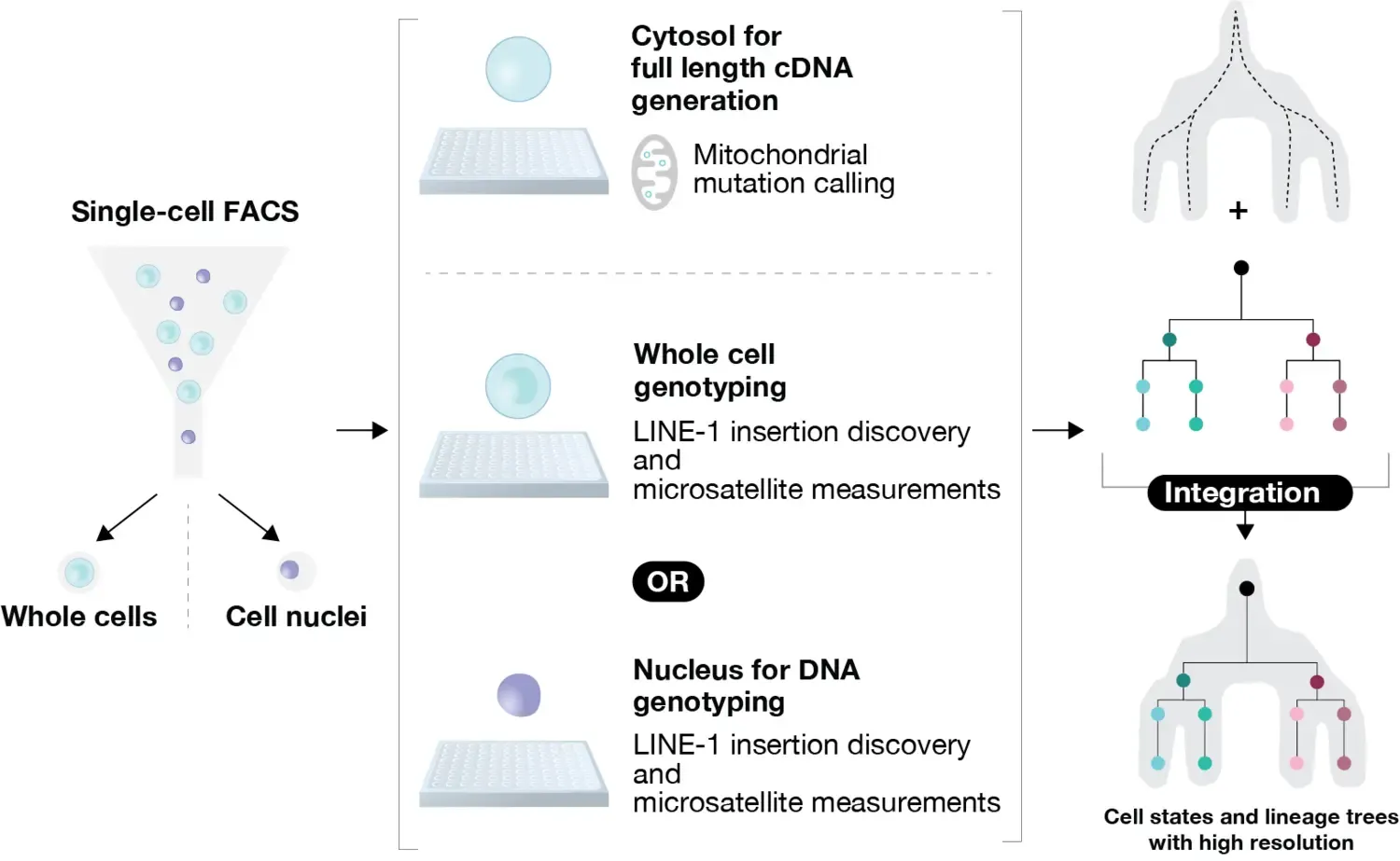
Knowing how cells are generated is key for our understanding of development, tissue homeostasis and regeneration, but is very challenging to study in humans. We plan to develop novel single-cell methodologies to study how cells are related and turn over in the human body, and apply these methods to study human hematopoiesis and neurogenesis. We aim to capture a combination of less explored sources of somatic variation in the human genome in single cells: X-chromosome inactivation, mitochondrial mutations, LINE-1 retrotransposition, and microsatellite mutations to resolve clonal relationships within these tissues. We envision our approach to unveil new insights for human developmental biology and enable new research directions.
Katarina Le Blanc

Cured but not well: Long term fatigue and cognitive dysfunction due to synaptopathy or brain GvHD?
Leukemia survivors suffer from severe fatigue and cognitive dysfunction.
Fatigue and cognition highly correlated with both a skewed immune repertoire in liquor and reduced factors involved in immune regulation, neurogenesis and synapse function and differentiation.We believe our patient cohort allows for mechanistic studies and hypothesize that activated microglia and alloreactive T-cells in the central nervous system (CNS)disrupt synaptic function.
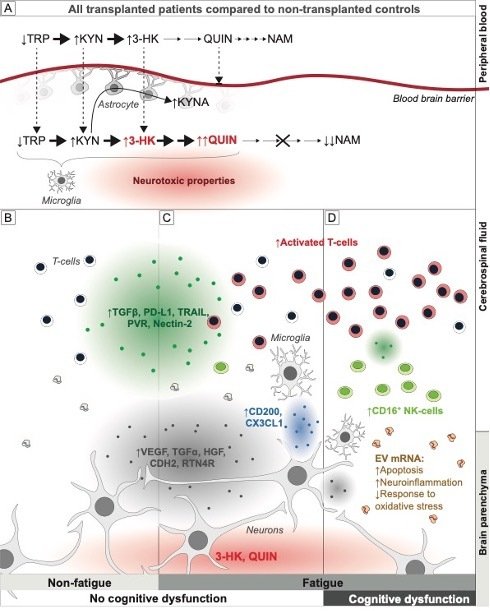
We have established a robust multiparameter flow cytometry panel for in depth analysis of the CNS immune compartment that we will combine with analyses of signs of activated perivascular stroma, possibly contributing to disruption of blood brain barrier integrity, permissive of alloreactive donor cell influx. In addition, we will measure immune cell activity in the CNS in vivo, known to associate with cognitive dysfunction in neuroinflammation.
If our hypothesis is correct, patients at risk benefit from neuroprotective treatment.
More research is performed in Katarina Le Blanc´s group.
Pete Williams

Switchable gene therapies for optic nerve protection and regeneration
There are no treatment strategies that simultaneously protect and repair the optic nerve following disease or injury. The goal of this StratRegen research program is to develop a clinically translatable treatment for glaucoma and other optic neuropathies via synergistic targeting of both neuroprotective and neuroregenerative mechanisms.
Specifically, my team will simultaneously target neuroprotection and axon regeneration to develop a comprehensive, titratable, single dose treatment for glaucoma via gene therapy by:
(1) Combining a regenerative gene therapy (phosphomimetic Protrudin) with a neuroprotective treatment that has progressed to Phase III clinical trials (oral nicotinamide treatment targeting NAD synthesis),
(2) Testing a novel dual gene therapy construct combining Protrudin (pro-regenerative) and full length human NMNAT2 (neuroprotective) to develop a one dose treatment with relevance for different stages of glaucoma,
(3) Exploiting the E. coli DHFR and human FKBP destabilising domains to develop a gene therapy product which is rapid, titratable, and switchable (i.e. ON/OFF) using topical or systemic antibiotics/ligands.
More research is performed in Pete Williams´s group.
Rula Zain

Nucleic-acid therapeutic reprogramming of tandem-repeat disease genes
There are more than 50 tandem-repeat expansion disorders (TRED) affecting humans, most of which are very severe and for which disease-reverting treatment is lacking. TREDs often cause neurodegeneration and share unusual characteristics including somatic repeat expansion leading to disease progression. We aim to study the cellular and molecular pathways that regulate the expansion of tandem-repeats.
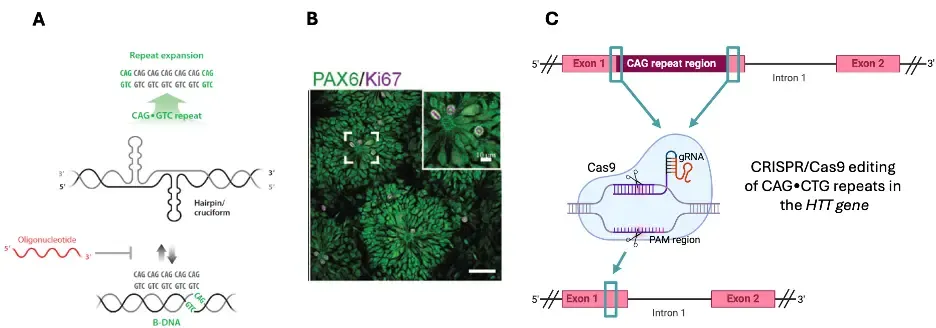
We will use stem cells of different origins with focus on Huntington’s disease (HD) and regenerative techniques including chemically modified DNA-targeting anti-gene oligonucleotides (A-GOs) and CRISPR/Cas gene editing. We aim to extend our nucleic acid-based therapeutic strategies to reprogram cells to prevent repeat expansion, the root cause of HD as well as several other TREDs.
More research is performed in Rula Zains group.
