Intracellular Calcium Oscillations
Calcium ions play a very important role for triggering the release of insulin from pancreatic beta-cells. The intracellular calcium concentration ([Ca2+]i) is regulated by a tight interplay between a number of processes that serve to increase or decrease [Ca2+]I. The temporal pattern of [Ca2+]I is oscillatory in beta-cells within the intact islet, both in vivo in the living organism, as well as in islets isolated in vitro.
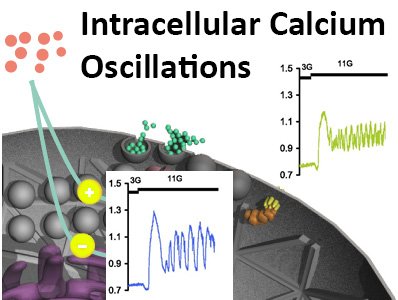
Regulation and coordination of [Ca2+]I oscillations within the intact islet
A constant glucose concentration of 10 mM given to intact islets typically evokes a slow wave electrical activity pattern on which action potentials, bursts, are superimposed. After the depolarization phase of the burst, that is associated with Ca2+ influx through voltage-gated L-type Ca2+ channels, there is a phase where action potentials are initiated from a plateau potential. During both these phases there is a substantial influx of Ca2+ that accumulates in the cytoplasm and in intracellular Ca2+ stores like endoplasmic reticulum (ER). Every burst is terminated by a rapid repolarization to slightly below the threshold potential. This repolarization is mediated by opening of Ca2+ activated K+ channels, where Ca2+ from ER plays an active role. Repolarization also leads to closure of L-type Ca2+ channels whereby [Ca2+]i decreases. Oscillating [Ca2+]i that is associated with islet beta-cell bursting electrical activity, we denote as ‘fast oscillations’. The [Ca2+]I oscillations are coordinated through intercellular connections between beta-cells in the intact pancreatic islet, leading to well-regulated exocytosis of insulin.
Significance of islet [Ca2+]I oscillations for diabetes
We have previously shown that islets from mouse models of premature ageing and models with disturbed mitochondrial function have a loss of function associated with a loss of fast [Ca2+]i oscillations. We have also shown that removal of the L-type calcium channel beta3 subunit is associated with a more robust phenotype where the fast oscillations remain until older age, compared with control mice. The exact mechanism behind this phenotype is not yet fully characterized.
Current projects
The topic of one project is the link between removal of the L-type calcium channel beta3 subunit and the mechanisms associated with maintenance of fast Ca2+ oscillations leading to an robust diabetes-resistant phenotype. In another project we are characterizing the three-dimensional functional network between islet beta-cell in vivo.
