Research
Manipulation of the p53 tumor suppressor pathway: from lab bench to clinic
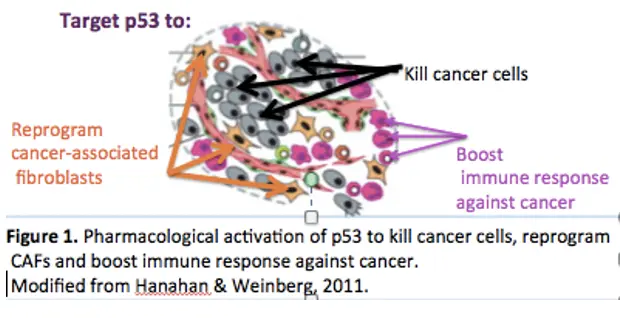
p53 is the major tumor suppressor which eliminates damaged or oncogene-expressing cells by activating transcription of genes inducing apoptosis, cell cycle arrest or senescence. p53 inactivation via mutations or enhanced degradation by MDM2 is the most frequent alteration in human cancers, which underscores the key role of p53 in combating cancer. Reinstatement of p53 by genetic means have demonstrated remarkable tumor suppression in animal models, including inhibition of aggressive metastatic lesions. This inspires the idea of developing small molecules reactivating p53 to fight cancer (Figure 1).
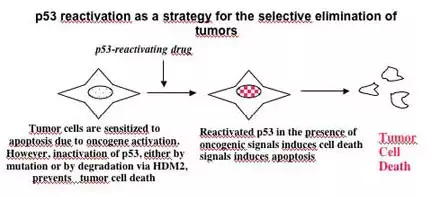
Depending on the type of p53 inactivation, there could be envisioned two major strategies of p53 reinstatement: restoring the function of mutant p53 and preventing p53 inactivation by MDM2 (Figure 2).
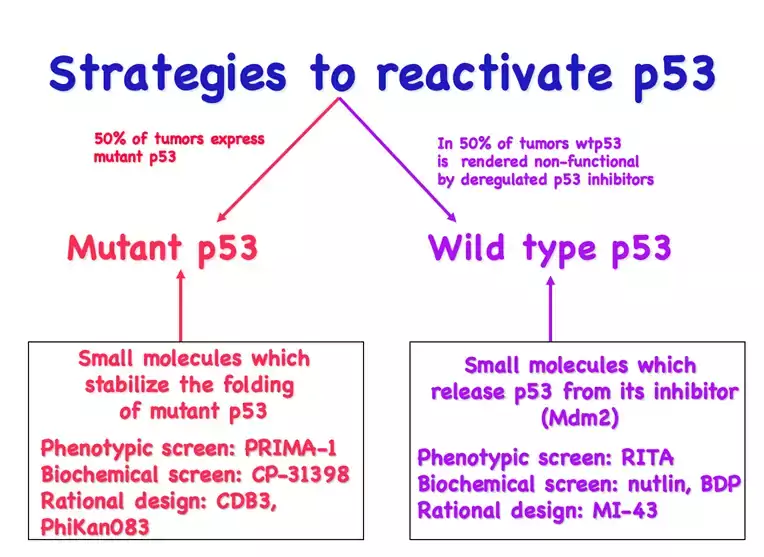
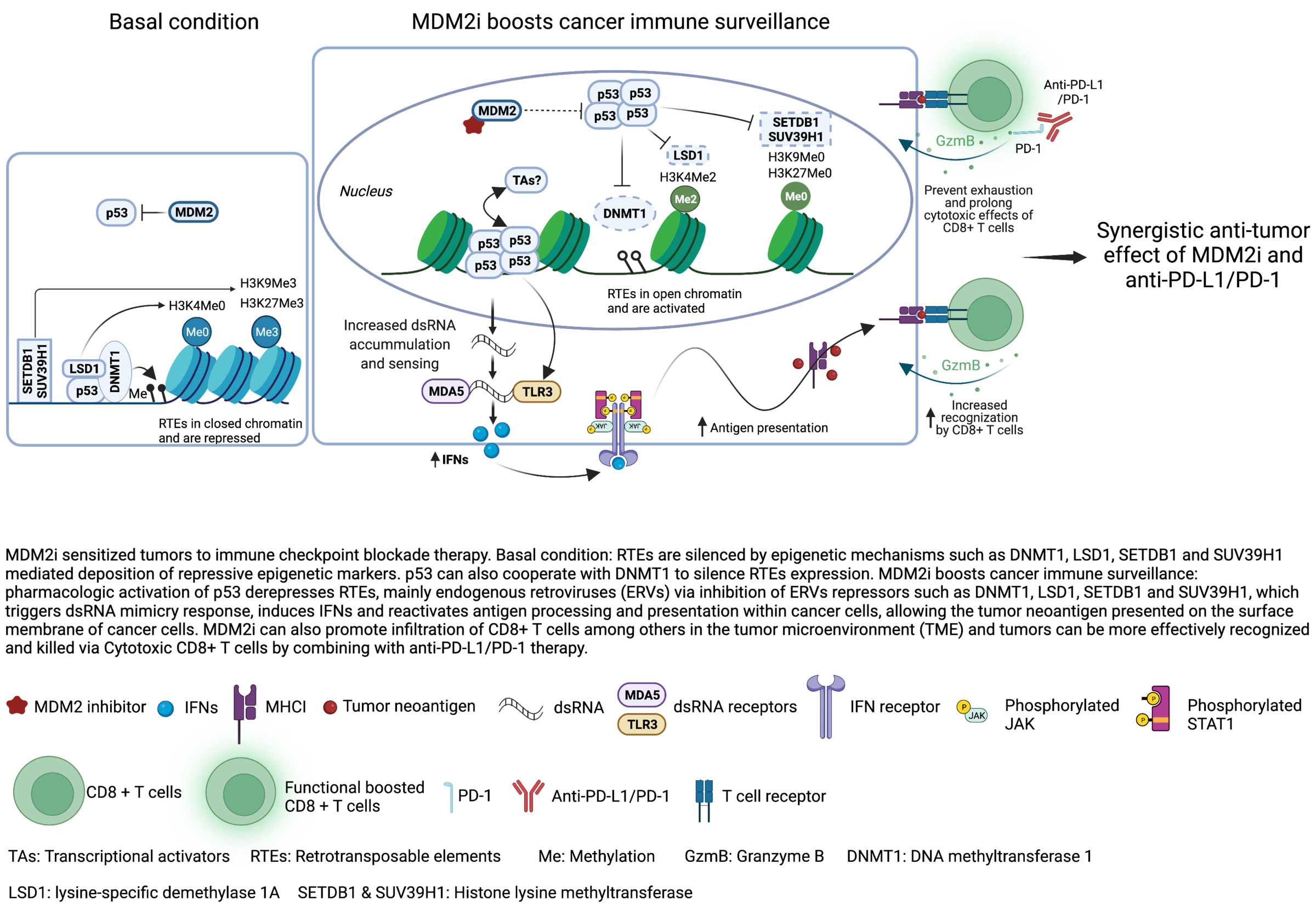
A number of MDM2 inhibitors reactivating wild type p53 have been discovered, including small molecule RITA discovered by us (Issaeva et al, Nature Medicine, 2004; Enge et al, Cancer Cell 2009; Grinkevich et al, Cancer Cell, 2009). Several of these are currently being tested in clinical trials, including derivatives of nutlin and the stapled peptide ATSP-7041, which are activating wild type (wt) p53 are in Phase I/II trials (www.cilicaltrials.gov, see also our review Sanz G et al, J Mol Cell Biol, 2019).
Mutant p53 is expressed in cancers, where it adopts an unfolded conformation resulting in loss of DNA binding and in some cases gaining oncogenic function. Thus, our hypothesis is to stabilize p53 conformation by small molecule to restore the DNA binding tumor suppressor function of p53. Since around 50% of all human tumors carry mutations in p53, it could be widely applicable in clinic. We have identified a small molecule PRIMA-1MET by screening NCI chemical library using cell-based assay (Bykov et al, Nature Medicine, 2002). PRIMA-1MET(commercial name APR-246, please see www.aprea.com) restores the active conformation and DNA binding of mutant p53 in cells and in vitro, re-activates the function of mutant p53 in tumor cells of different origin and suppreses the growth of human xenograft tumors in mice.
APR-246 has been tested in patients in Phase I clinical trial and in March 2020 APR-246 received Fast Track and Breakthrough Therapy designations from FDA based on outstanding results of Phase II trial in MDS patients. These prompted the start of Phase III randomized trial in MDS.

In light of clinical developments of p53-targeting therapies, it is imperative to rationally design combinatorial treatments (since monotherapy cannot cure cancer) and find biomarkers to stratify patients. To achieve these, we need to get a deep understanding of the molecular mechanisms and pathways affected by p53 and p53-reactivating compounds.
If you would like to learn more about our research (or just to drop in to say hello!) you are welcome to visit us at Biomedicum, Quarter C8.
 Photo: Chokniti Khongchum
Photo: Chokniti KhongchumMake a donation to our research at MTC
Your support means a lot to our success. This allows us to go further in our efforts to improve human health through research and education.
Read here how you can make a donation via Swish.