Fredrik Lanner
Fredrik Lanner undertook his PhD thesis at the department of Cell and Molecular Biology Karolinska Institutet followed by postdoctoral research in Janet Rossants lab at The Hospital for Sick Children, Toronto. Having returned to Karolinska Institutet, Sweden, he has established his independent research lab exploring how pluripotent stem cells are regulated in the human embryo and how we best could use embryonic stem cells in stem cell based reparative medicine.

To start exploring early human development and pluripotency, Fredrik Lanner and his lab performed a single cell transcriptional roadmap of human preimplantation development (Petropoulos et. al., Cell 2016). Subsequently the lab identified cell surface markers for naïve and primed pluripotent stem cells (Collier et. al., Cell Stem Cell 2017). Adult oogonial stem cells have been suggested to reside in the adult human ovary with a therapeutic potential to treat infertility. The laboratory have through two papers refuted such claims and through single cell sequencing mapped the cell types of the human ovarian cortex and established that the putative stem cells do not harbor the capacity to make oocytes and are instead mistaken perivascular cells (Zhang et. al., Nature Medicie 2015, Wagner et. al., Nature Communications 2020).
In order to use embryonic stem cells in reparative medicine the cells must be established and cultured under Good Manufacturing Practice (GMP). For this purpose the lab have a new cell line (KARO1) within the GMP manufacturing facility at Karolinska University Hospital. Another hurdle that needs to be addressed is the immunological barrier when transplanting allogeneic cells. For that reason the laboratory have explored the use of genome editing to evade the immune system by eliminating HLA presentation (Petrus-Reurer et. al., Stem Cell Reports 2020).
Finally, building on our established xeno-free and defined protocol to generate stem cell derived derived retinal cells (Plaza-Reyes et al 2016 Stem Cell Reports) we have identified cell surface markers for retinal pigmented epithelial cells and established a streamlined protocol for RPE differentiation (Petrus-Reurer et. al., Stem Cell Transl Med. 2020, Plaza Reyes et. al., Nature Communication 2020) which now puts us in the position to initiate clinical trial together with St Erik Eye Hospital to test our stem cell based treatment strategy of age-related macular degeneration.
Selected Publications
Petrus-Reurer, S., Kumar, P., Padrell Sánchez, S., Aronsson, M., André, H., Bartuma, H., Plaza Reyes, A., Nandrot, E. F., Kvanta, A., & Lanner, F. (2020). Preclinical safety studies of human embryonic stem cell-derived retinal pigment epithelial cells for the treatment of age-related macular degeneration. Stem Cells Translational Medicine, 9(8), 936–953. https://doi.org/10.1002/sctm.19-0396
Petrus-Reurer, S., Winblad, N., Kumar, P., Gorchs, L., Chrobok, M., Wagner, A. K., Bartuma, H., Lardner, E., Aronsson, M., Plaza Reyes, Á., André, H., Alici, E., Kaipe, H., Kvanta, A., & Lanner, F. (2020). Generation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells Lacking Human Leukocyte Antigen Class I and II. Stem Cell Reports, 14(4), 648–662. https://doi.org/10.1016/j.stemcr.2020.02.006
Plaza Reyes, A., Petrus-Reurer, S., Padrell Sánchez, S., Kumar, P., Douagi, I., Bartuma, H., Aronsson, M., Westman, S., Lardner, E., André, H., Falk, A., Nandrot, E. F., Kvanta, A., & Lanner, F. (2020). Identification of cell surface markers and establishment of monolayer differentiation to retinal pigment epithelial cells. Nature Communications, 11, 1609. https://doi.org/10.1038/s41467-020-15326-5
Wagner, M., Yoshihara, M., Douagi, I., Damdimopoulos, A., Panula, S., Petropoulos, S., Lu, H., Pettersson, K., Palm, K., Katayama, S., Hovatta, O., Kere, J., Lanner, F., & Damdimopoulou, P. (2020). Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nature Communications, 11(1), 1147. https://doi.org/10.1038/s41467-020-14936-3
Collier, A. J., Panula, S. P., Schell, J. P., Chovanec, P., Plaza Reyes, A., Petropoulos, S., Corcoran, A. E., Walker, R., Douagi, I., Lanner, F., & Rugg-Gunn, P. J. (2017). Comprehensive Cell Surface Protein Profiling Identifies Specific Markers of Human Naive and Primed Pluripotent States. Cell Stem Cell, 20(6), 874–890. https://doi.org/10.1016/j.stem.2017.02.014
Petropoulos, S., Edsgärd, D., Reinius, B., Deng, Q., Panula, S. P., Codeluppi, S., Plaza Reyes, A., Linnarsson, S., Sandberg, R., & Lanner, F. (2016). Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation Embryos. Cell, 165(4). https://doi.org/10.1016/j.cell.2016.03.023
Plaza Reyes, A., Petrus-Reurer, S., Antonsson, L., Stenfelt, S., Bartuma, H., Panula, S., Mader, T., Douagi, I., André, H., Hovatta, O., Lanner, F., & Kvanta, A. (2016). Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model. Stem Cell Reports. https://doi.org/10.1016/j.stemcr.2015.11.008
Zhang, H., Panula, S., Petropoulos, S., Edsgärd, D., Busayavalasa, K., Liu, L., Li, X., Risal, S., Shen, Y., Shao, J., Liu, M., Li, S., Zhang, D., Zhang, X., Gerner, R. R., Sheikhi, M., Damdimopoulou, P., Sandberg, R., Douagi, I., … Liu, K. (2015). Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nature Medicine, 21(10), 1116–1118. https://doi.org/10.1038/nm.3775
 Photo: Banushree Kumar
Photo: Banushree KumarFredrik Lanner and Simon Elsässer's new publication in Nature Cell Biology
Fredrik Lanner and Simon Elsässer joined forces to identify the function of Polycomb Repressor Complex 2 (PRC2) as an epigenetic barrier in maintaining the separation of embryonic and extraembryonic lineages. In this study, the two laboratories elucidate how distinct cell types are established during early human development, which increase our understanding of human development and early pregnancy, and provide an avenue to study disease condition in cellular models.
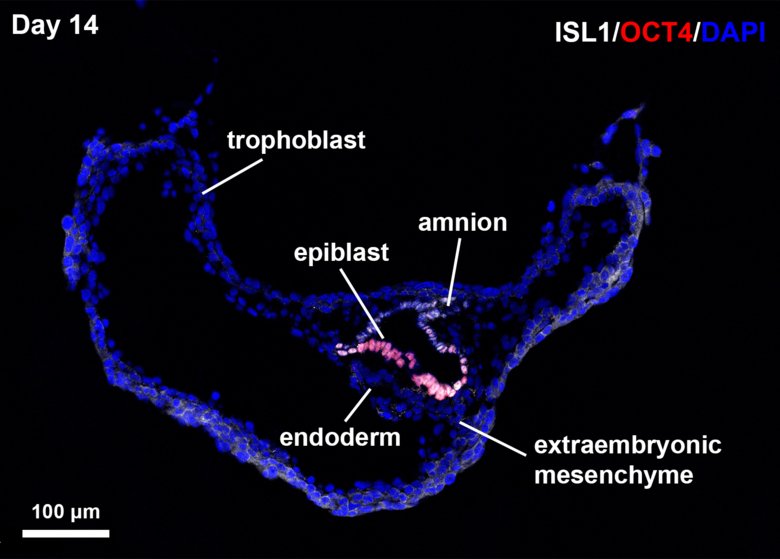 Photo: Fredrik Lanner
Photo: Fredrik LannerFredrik Lanner’s new publication in Nature Communications
In this study, The Lanner lab reveals an unexpected role of the amnion for induction of mesoderm during gastrulation. Through transcriptional profiling of in vitro cultured non-human primate (NHP) blastocysts, they identify that ISL1 is a primate specific marker of the amnion and that genetic targeting through CRISPR/Cas9 disrupts mesoderm formation. The study provides a detailed transcriptional atlas of the implantation stage primate embryo from day 10-14 which can be easily browsed at https://www.nhp-embryo.net
 Photo: Eszter Posfai
Photo: Eszter PosfaiFredrik Lanner has a new publication in Nature Cell Biology
Stem cell research is fundamental for reparative medicine, which with the help of the body's cells, recreates and heals important organs. Now, Fredrik Lanner’s team, together with researchers at SickKids in Canada and KU Leuven in Belgium, have developed a method for defining the most general type of stem cells, that can develop into all cell types in the body. The study of totipotent stem cells in mice has been published in Nature Cell Biology.
 Photo: Hallbauer&Fioretti,Keegan Houser
Photo: Hallbauer&Fioretti,Keegan HouserKI researchers' excitement for the Nobel Prize in Chemistry 2020
Emmanuelle Charpentier and Jennifer A. Doudna were awarded the Nobel Prize in Chemistry 2020 for discovering one of gene technology’s sharpest tools: the CRISPR/Cas9 genetic scissors. This technology is revolutionary as it provides a quick and precise method in genome editing. Fredrik Lanner and Zongli Zheng are some of the KI researchers who use this technology in their research. Let's learn more about this powerful award-winning technology.
 Photo: Ulf Sirborn
Photo: Ulf SirbornKI, St. Erik Eye Hospital and Novo Nordisk collaborate to find a cure for age-related macular degeneration
Dr Fredrik Lanner and Prof Anders Kvanta, together with their research teams at KI and St. Erik Eye Hospital have entered a collaboration with Novo Nordisk A/S to develop a new treatment for age-related macular degeneration. The aim is to develop a completely new cell therapy for this common but currently incurable eye disease.
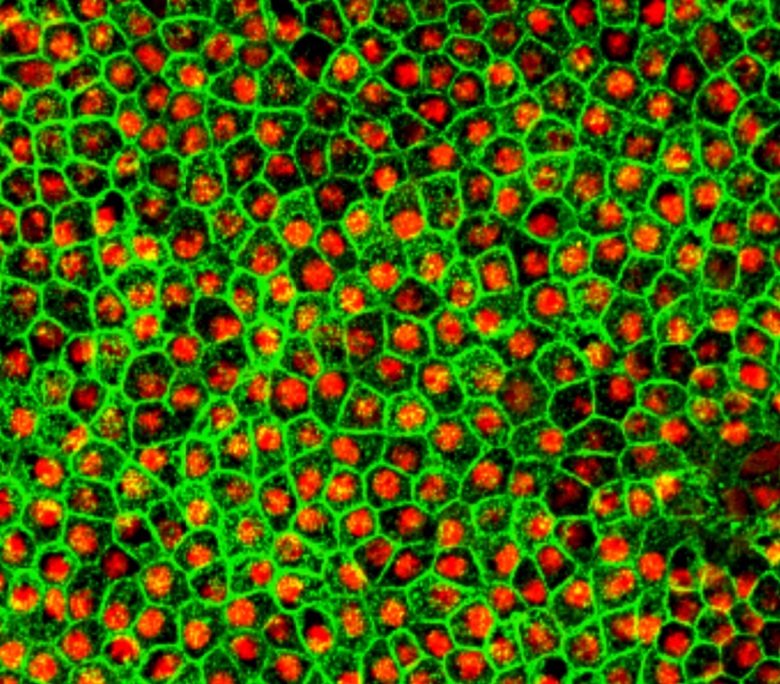 Photo: Fredrik Lanner
Photo: Fredrik LannerFredrik Lanner has new publications in both Nature Communications and Stem Cell Reports on 30 MAR
In collaboration with Anders Kvanta at St Erik Eye Hospital in Sweden, Fredrik Lanner's team have discovered a way to refine the production of retinal cells from embryonic stem cells for treating blindness in the elderly. Using the CRISPR/Cas9 gene editing, they have also managed to modify the cells so that they can hide from the immune system to prevent rejection. The studies are published in the scientific journals Nature Communications and Stem Cell Reports.
