AROGYA: Healthy Aging with HIV
We have initiated an interdisciplinary study, called AROGYA which means wellbeing in Sanskrit. The overall aims of the AROGYA project are to use complimentary inter-disciplinary expertise to unravel the physiological and molecular pathways that underline the premature aging of the immune system (immune-aging) and to potentially generate novel therapeutic approaches for age-related diseases with a focus on the people who are living with HIV (PLHIV).
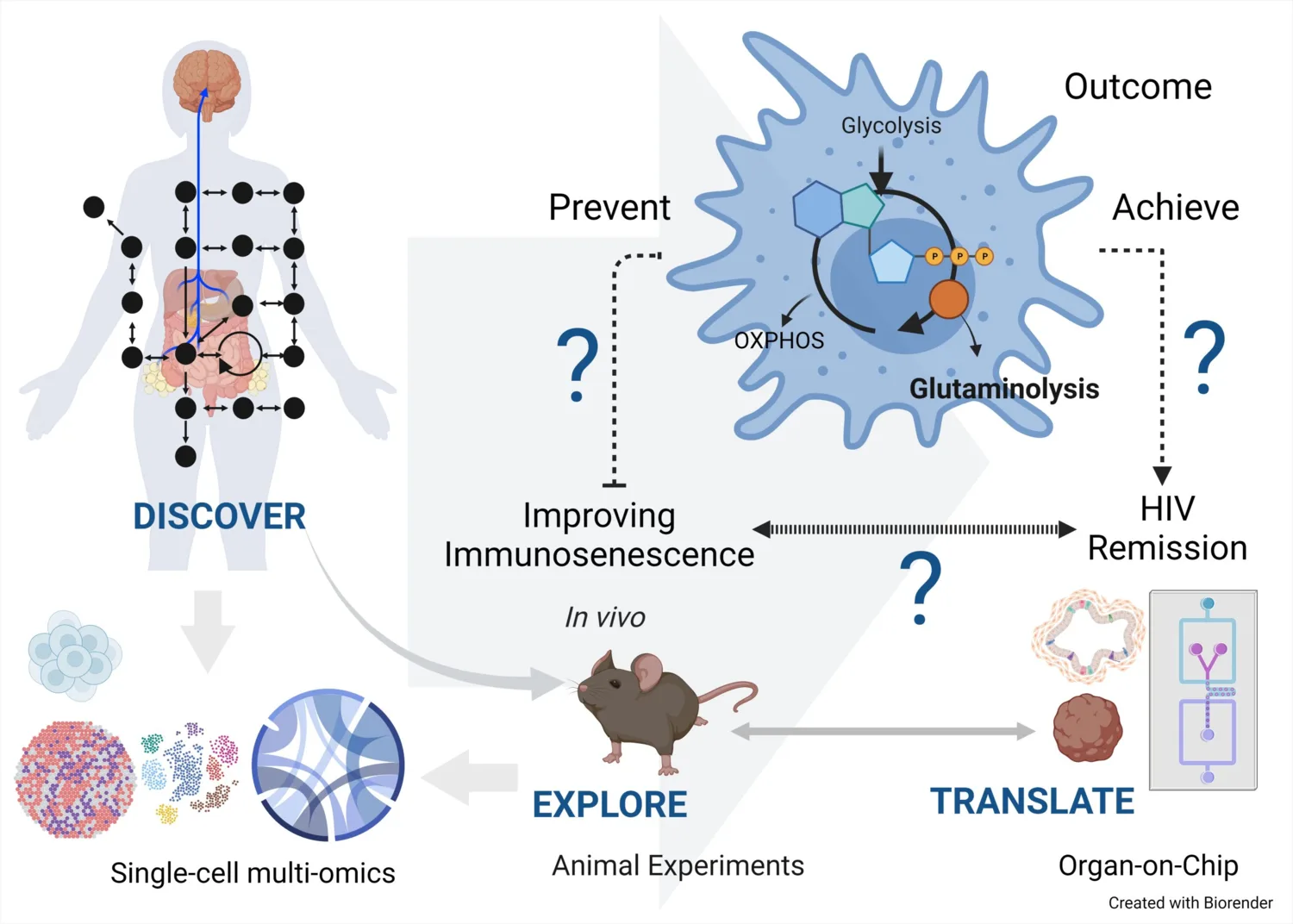
Healthy aging with HIV has become an increasingly relevant and achievable goal, thanks to advancements in medical care and antiretroviral therapies. As individuals with HIV live longer, the focus has shifted to promoting overall well-being and addressing age-related health concerns. Comprehensive healthcare strategies now emphasize controlling the virus and managing other health factors, such as cardiovascular health, bone density, mental health, and social well-being.
The application of interdisciplinary science, specifically the integration of multi-omics systems biology and advanced technology like organ-on-chip, holds immense promise in understanding and promoting healthy aging. To explore the intricate molecular mechanisms underlying the aging process at a systemic level, we use multi-omics systems biology that involves the comprehensive analysis of various biological data sets, such as transcriptomics, proteomics, metabolomics, microbiome, and more, from well-defined clinical cohorts from both high and low-income settings.
Objectives
The primary objectives of the AROGYA study include leveraging interdisciplinary expertise to decode the physiological and molecular mechanisms driving the premature aging of the immune system, known as immune aging. The project focuses on developing innovative therapeutic strategies for age-associated diseases, with particular attention to individuals living with HIV (PWH).
- Create and apply innovative technologies for isolating, detecting, and profiling single cells, enhancing high-throughput basic and translational research.
- Establish a frame of reference to identify new biomarkers of immune aging through proteotranscriptomic studies of specific cell subsets, which could be pivotal for clinical interventions.
- Detect changes in metabolic patterns that offer therapeutic opportunities to intervene in the immune aging process through metabolic manipulation.
- Confirm the new biomarkers discovered in -omics studies and elucidate the cellular mechanisms underlying immune aging in PWH.
Publications
- Transcriptomics age acceleration in prolonged treated HIV infection.
Mikaeloff F, Gelpi M, Escos A, Knudsen AD, Høgh J, Benfield T, de Magalhães JP, Nielsen SD, Neogi U
Aging Cell 2023 Oct;22(10):e13951 - Role of myeloid cells in system-level immunometabolic dysregulation during prolonged successful HIV-1 treatment.
Svensson Akusjärvi S, Krishnan S, Ambikan AT, Mikaeloff F, Munusamy Ponnan S, Vesterbacka J, Lourda M, Nowak P, Sönnerborg A, Neogi U
AIDS 2023 Jun;37(7):1023-1033 - Network-based multi-omics integration reveals metabolic at-risk profile within treated HIV-infection.
Mikaeloff F, Gelpi M, Benfeitas R, Knudsen AD, Vestad B, Høgh J, Hov JR, Benfield T, Murray D, Giske CG, Mardinoglu A, Trøseid M, Nielsen SD, Neogi U
Elife 2023 Feb;12(): - Genome-scale metabolic models for natural and long-term drug-induced viral control in HIV infection.
Ambikan AT, Svensson-Akusjärvi S, Krishnan S, Sperk M, Nowak P, Vesterbacka J, Sönnerborg A, Benfeitas R, Neogi U
Life Sci Alliance 2022 Sep;5(9):e202201405 - Peripheral blood CD4+CCR6+ compartment differentiates HIV-1 infected or seropositive elite controllers from long-term successfully treated individuals.
Svensson Akusjärvi S, Krishnan S, Jütte BB, Ambikan AT, Gupta S, Rodriguez JE, Végvári Á, Sperk M, Nowak P, Vesterbacka J, Svensson JP, Sönnerborg A, Neogi U
Commun Biol 2022 Apr;5(1):357 - Integrative proteo-transcriptomic and immunophenotyping signatures of HIV-1 elite control phenotype: A cross-talk between glycolysis and HIF signaling.
Akusjärvi SS, Ambikan AT, Krishnan S, Gupta S, Sperk M, Végvári Á, Mikaeloff F, Healy K, Vesterbacka J, Nowak P, Sönnerborg A, Neogi U
iScience 2022 Jan;25(1):103607 - Trans cohort metabolic reprogramming towards glutaminolysis in long-term successfully treated
HIV-infection.
Mikaeloff F, Svensson Akusjärvi S, Ikomey GM, Krishnan S, Sperk M, Gupta S, Magdaleno GDV, Escós A, Lyonga E, Okomo MC, Tagne CT, Babu H, Lorson CL, Végvári Á, Banerjea AC, Kele J, Hanna LE, Singh K, de Magalhães JP, Benfeitas R, Neogi U
Commun Biol 2022 Jan;5(1):27 - Integrative Lipidomics and Metabolomics for System-Level Understanding of the Metabolic Syndrome in Long-Term Treated HIV-Infected Individuals.
Olund Villumsen S, Benfeitas R, Knudsen AD, Gelpi M, Høgh J, Thomsen MT, Murray D, Ullum H, Neogi U, Nielsen SD
Front Immunol 2021 ;12():742736 - Fecal Metabolome Signature in the HIV-1 Elite Control Phenotype: Enrichment of Dipeptides Acts as an HIV-1 Antagonist but a Prevotella Agonist.
Sperk M, Ambikan AT, Ray S, Singh K, Mikaeloff F, Diez RC, Narayanan A, Vesterbacka J, Nowak P, Sönnerborg A, Neogi U
J Virol 2021 Aug;95(18):e0047921 - Distinct lipid profile, low-level inflammation, and increased antioxidant defense signature in HIV-1 elite
control status.
Sperk M, Mikaeloff F, Svensson-Akusjärvi S, Krishnan S, Ponnan SM, Ambikan AT, Nowak P, Sönnerborg A, Neogi U
iScience 2021 Feb;24(2):102111 - The central role of the glutamate metabolism in long-term antiretroviral treated HIV-infected individuals with metabolic syndrome.
Gelpi M, Mikaeloff F, Knudsen AD, Benfeitas R, Krishnan S, Svenssson Akusjärvi S, Høgh J, Murray DD, Ullum H, Neogi U, Nielsen SD
Aging (Albany NY) 2021 Oct;13(19):22732-22751 - Sub-attomole detection of HIV-1 using padlock probes and rolling circle amplification combined with microfluidic affinity chromatography.
Soares RRG, Varela JC, Neogi U, Ciftci S, Ashokkumar M, Pinto IF, Nilsson M, Madaboosi N, Russom A
Biosens Bioelectron 2020 Oct;166():112442
Collaborators
AROGYA brings together clinicians, basic scientists and translational medical researchers with technology experts and engineers.
Principal Investigator

Ujjwal Neogi, PhD
Karolinska Institutet, Sweden
Ujjwal is an Associate Professor of Virology and Group Leader in Department of Laboratory Medicine, Karolinska Institutet. He received the Swedish Research Council establishment grant 2017 to understand the HIV-1 disease control mechanism using a multi-omics system biology approach. His interest is to understand the host immune defenses against viral infections using multi-omics system biology studies and mechanistic understanding through experimental models.

Collaborators
Susanne Dam Poulsen, MD, DMSc
Rigshospitalet and University of Copenhagen, Denmark
Susanne Dam Poulsen is a Professor of Infectious Diseases at the University of Copenhagen, Denmark, and has decade-long experience in treating people living with HIV (PWH) with different co-morbidities. She is the custodian of one of the largest European bio-banked HIV aging cohorts named the Copenhagen Comorbidity in HIV-infection (COCOMO), the study for a longitudinal, non-interventional assessment of non-AIDS comorbidity in HIV infection in Denmark.

Dasja Pajkrt, MD, PhD
Amsterdam University Medical Centers, The Netherlands
Dasja Pajkrt is a Viral Pediatric Infectious Diseases Professor at the Amsterdam University Medical Centers (Amsterdam UMC), The Netherlands. She is the Principal Investigator (PI) of the Department of Pediatric Infectious Diseases and the study “Neurological, cardiovascular, visual, and neurocognitive performance in pediatric HIV-1- infected patients as compared to healthy controls (NOVICE)”. Dasja leads the research group OrganoVIR Labs, where clinicians, virologists, and tissue engineers work together to develop innovative animal-free models for virus research.

Adithya Sridhar, PhD
Amsterdam University Medical Centers, The Netherlands
Adithya Sridhar is a senior scientist at the OrganoVIR Labs at the Amsterdam University Medical Centers (Amsterdam UMC), The Netherlands. He has a degree in Biomedical Engineering from the University of Oxford and did his PhD in the lab-on-chip group of Prof. van den Berg at the University of Twente. He has over ten years of experience developing complex in vitro cell culture models. As a Senior Scientist at the Amsterdam UMC, he has set airway, gut, and brain organoid models for virology.

Yasir Ahmed Syed, PhD
Cardiff University, United Kingdom
Yasir Ahmed Syed is a Lecturer in Neuroscience at Cardiff University, UK. Syed lab is to define the biological basis of neurodevelopmental and neuropsychiatric disorders. Using the patient-derived pluripotent stem cells and differentiating them into multiple neural linage cells and organoids in vitro, the group employs a combination of cellular, genetic, electrophysiological, behavioral, and material science approaches to understand the mechanisms of disease initiation and progression, and ultimately develop novel and reliable drug targets. Within this project, we aim to identify the contribution of neuroinflammation to the pathogenesis of neurodevelopmental disorders and psychiatric phenotypes in PWH.

Piotr Nowak, MD, PhD
Karolinska Institutet, Sweden
Piotr is an Associate Professor at Karolinska Institutet and senior consultant, director of HIV Clinical Research Unit and the Therapeutic unit for Microbiota Transplantation at Karolinska University Hospital. His main research interest is to understand the role of the microbiome in chronic infections like HIV /HCV and Mycobacterium tuberculosis and the mechanisms behind the success of microbiome modulation in the clinic through fecal microbial transplantation.

Funding
The study is funded by Vetenskapsrådet (the Swedish Research Council).
The study is also partially funded by:
- The Swedish Physicians Against AIDS
- Åke Wibergs Stiftelse
