Research description
Oxygen Sensing and Cancer
How do we cope with oxygen deprivation (hypoxia) in health and disease?
Oxygen sensors enable the cell to adapt to low-oxygen environments and drive metabolic adaptation, but are also critical for normal development and apoptosis. Hypoxia is a hallmark of cardiovascular disease and cancer, which are the leading causes of death worldwide.
Our research concerns the mechanisms of how alterations in oxygen-sensing pathways can lead to cancer. We are interested how we adapt to hypoxia at the cellular level, and using that knowledge to combat diseases, such as cancer. Oxygen sensing is mediated partly via prolyl hydroxylases that require molecular oxygen for enzymatic activity.
Our work focuses on the identification of novel oxygen-sensing pathways that are implicated in malignant transformation, with focus on cancer arising from the sympathoadrenal lineage, such as neuroblastoma and pheochromocytoma.
Exploring drivers of intratumor heterogeneity and phenotypic plasticity
Why are advanced cancers eventually acquiring resistance to targeted therapies and relapse?
Acquired resistance is the direct consequence of pre-existing intratumor heterogeneity. Systematic characterization of dynamic properties of intratumor heterogeneity and phenotypic plasticity can guide treatment strategies and improve clinical outcome for cancer patients.
Currently, our laboratory developed a new analytic and experimental approach based on single nuclei transcriptomics and mass spectrometry to explore intra-tumor heterogeneity and plasticity in childhood neuroblastoma and pheochromocytoma. We generated novel tumor mouse model systems and include human tumors to perform comparative differentiation trajectory analysis, to make predictions and, finally, to perform preliminary validations of envisioned tumor differentiation strategies.
We are exploring embryonic cell state transitions during sympatho-adrenal development that enables us to identify non-mutational drivers of intratumor heterogeneity.
Exploring clinical heterogeneity and outcome in high-risk childhood neuroblastoma
Childhood neuroblastoma has a remarkable variability in outcome. Age at diagnosis is one of the most important prognostic factors, with children less than 1 year old having favorable outcomes.
We study single-cell and single-nuclei transcriptomes of neuroblastoma with different clinical risk groups and stages, including healthy adrenal gland. We compare tumor cell populations with embryonic mouse sympatho-adrenal derivatives, and post-natal human adrenal gland. We provide evidence that low and high-risk neuroblastoma have different cell identities, representing two disease entities. Low-risk neuroblastoma presents a transcriptome that resembles sympatho- and chromaffin cells, whereas malignant cells enriched in high-risk neuroblastoma resembles a subtype of TRKB+ cholinergic progenitor population identified in human post-natal gland. Analyses of these populations reveal different gene expression programs for worst and better survival in correlation with age at diagnosis.
Our findings reveal two cellular identities and a composition of human neuroblastoma tumors reflecting clinical heterogeneity and outcome.
Our methods
Imaging
- RNA scope, IHC,
- Spatial techniques: In Situ Sequencing (ISS) in collaboration with Mats Nilson lab at Scilife
Bioinformatics
- Single nuclei RNAseq
- RNA velocity to explore trajectory analysis during tumor progression
- Proteomics analyses by nanoLC-MS/MS
Tumor mouse models
- Generating novel neuroblastoma and pheochromocytoma mouse models
- Lineage tracing
Biochemistry
- Hydroxylation assays
- Intro translation
- S35 capture pulldowns
- Seahorse to study mitochondrial metabolism
- shRNA and CRISPR/Cas9 lentiviral transduction
Single Cell Resources
Explore deep-sequence full-length coverage RNA from single cell and nuclei
- of the post-natal human gland,
- of postnatal mouse adrenal gland and
- of human neuroblastoma tumors across different risk groups
Funding
- H2020 European Research Commission ERC-2019-SyG
- Knut och Alice Wallenbergs Stiftelse
- Swedish Research Council (VR)
- Swedish Childhood Cancer Foundation (BCF)
- Swedish Cancer Society (CF)
- Para Difference Foundation
- Gösta Fraenckels stiftelse för medicinsk forskning
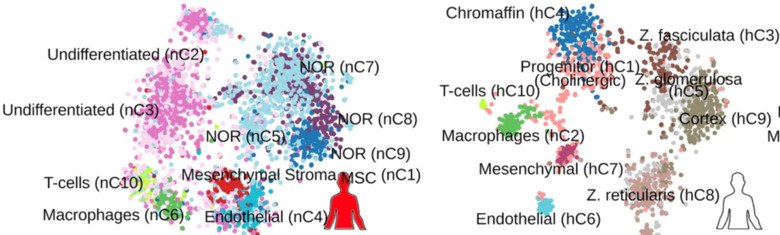 Photo: Susanne Schlisio group
Photo: Susanne Schlisio groupSingle Cell Resources
Explore deep-sequence full-length coverage RNA from single cell and nuclei of the post-natal human gland, postnatal mouse adrenal gland and human neuroblastoma tumors across different risk groups.
Follow us on social media
on Twitter



