Pre-GMP facility - our offer
The pre-GMP facility ensures safe and efficient translation of Advanced Therapy Medicinal Products (ATMPs) from the research laboratory to clinical application.
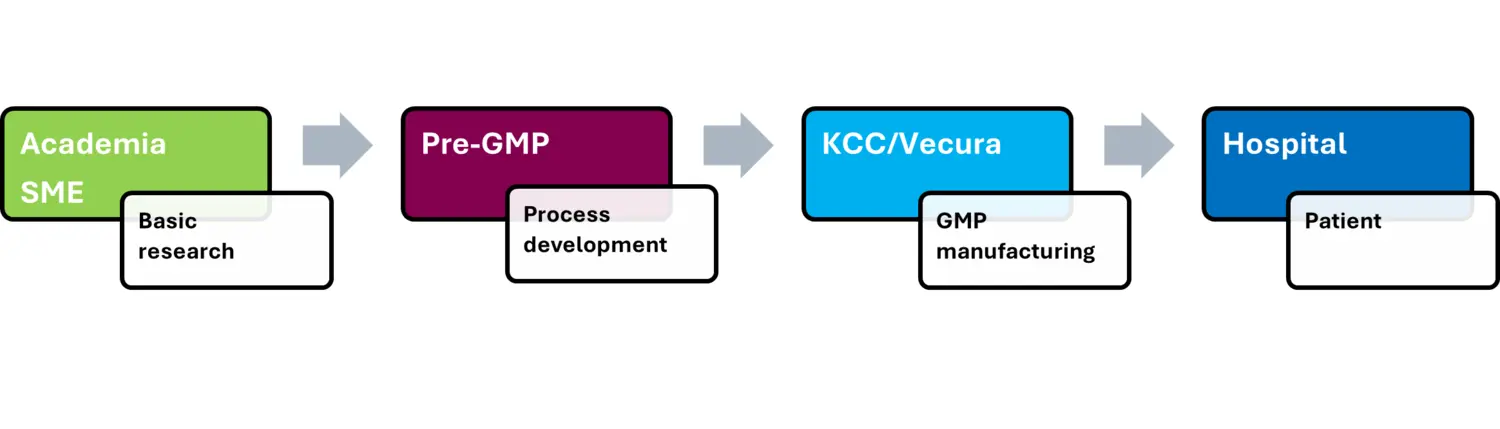
Translation of Advanced Therapy Medicinal Products (ATMPs) from the research laboratory to clinical application.
Photo: Gustaf Ahlén
At the pre-GMP facility, you have the opportunity to:
- Gain foundational knowledge of GMP (Good Manufacturing Practice) production.
- Providing support in project planning, process development, process and instrument training, SOP creation, and technology transfer.
- Convert research protocols into standardized operational procedures (SOPs) under GMP-like conditions.
- Conduct process development by creating SOPs and workflows that can be seamlessly transferred to a GMP facility.
- Perform validation runs that do not require full GMP compliance.
- Serve as a valuable resource for academia, healthcare, and industry.
