About Pre-GMP facility
Serving as a vital infrastructure for process development, the pre-GMP facility supports academia, healthcare, and private sector initiatives. It bridges the gap between research discoveries and pharmaceutical production by enabling rigorous testing of methods and detailed scrutiny of processes. This ensures the development of efficient and robust workflows that deliver high-quality products while prioritizing patient safety.

The ANA Futura pre-GMP facility receives core facility and strategic investment funding from Karolinska Institutet and is an integral part of the Karolinska ATMP Center.
Premises
The pre-GMP core facility is located at ANA Futura, Campus Flemingsberg - a newly renovated laboratory and office complex that houses over 300 world-leading scientists, clinicians, and teachers. Situated adjacent to Karolinska University Hospital, the facility is uniquely positioned to address the needs of patients requiring innovative therapies.
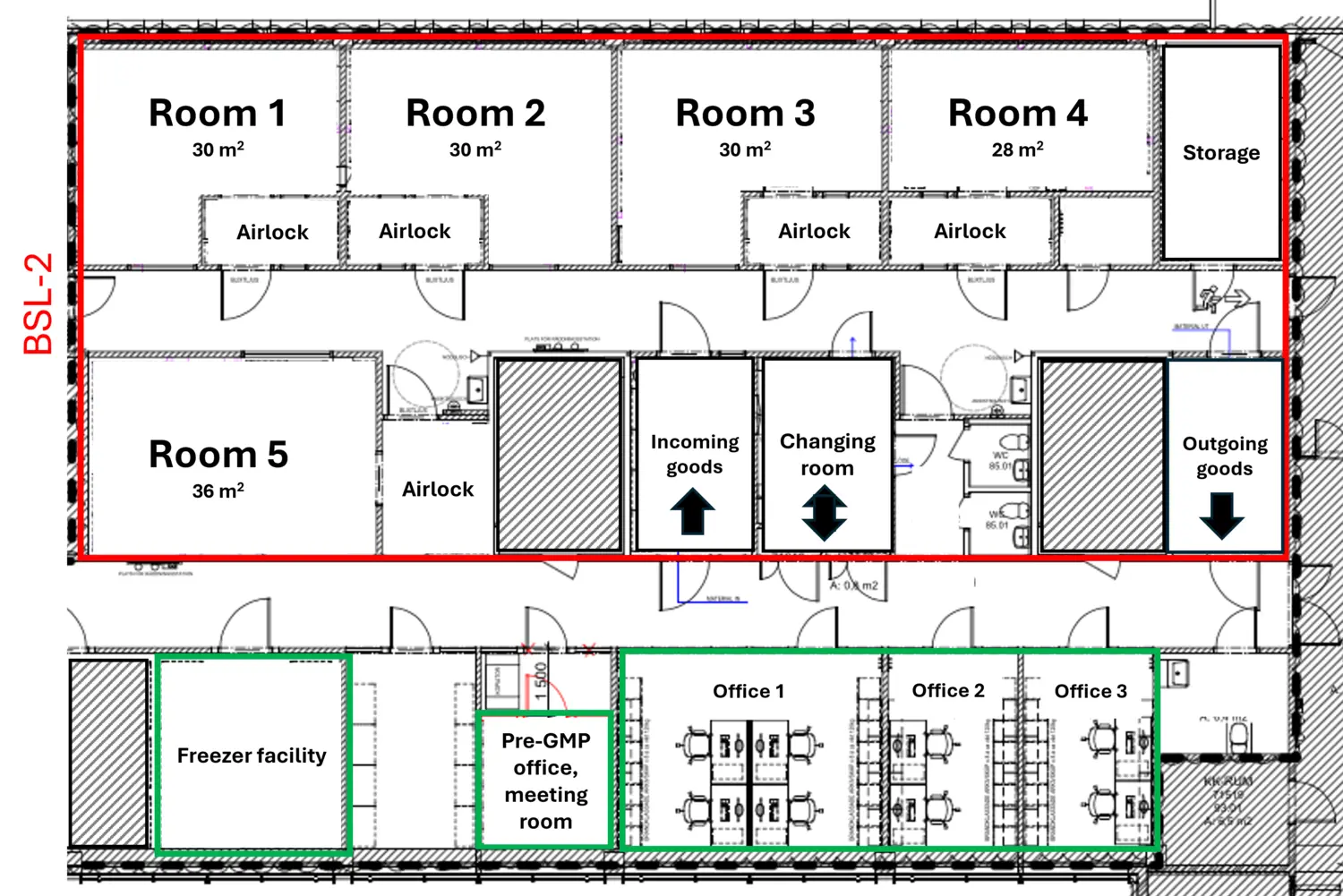
Spanning approximately 500 m², the pre-GMP facility features state-of-the-art laboratory space. It includes five cleanroom laboratories within a BSL-2 environment, as well as offices, storage areas, and a freezer facility.
Contact information
E-mail:
pregmp@ki.se
Phone number
08-524 838 57 and 070-268 97 58
Address:
Alfred Nobels Allé 8, 7th floor, Flemingsberg.
Staff and support
Gustaf Ahlén
Managing DirectorDaniela Nascimento Silva
Research SpecialistLars Frelin
ANA Futura facility manager
Service Team ANA Futura
Pre-GMP Steering Committee
- Matti Sällberg (Chairman)
- Gustaf Ahlén (Managing Director)
- Hans-Gustaf Ljunggren
- Evren Alici
- Pontus Blomberg, Director of KCC and Vecura
