
Close
Developmental and Translational Neurobiology – Cristiana Cruceanu's research group
Our group's research seeks to characterize how the environment impacts human brain development, and how prenatal exposures can shape mental health outcomes.
Part of the:

Latest news
 Photo: Creative Commons CC0
Photo: Creative Commons CC0Make a donation to our research
Your support means a lot to the success of research. This allows us to go further in our efforts to improve human health through research and education.
Social media
Our research
Image:
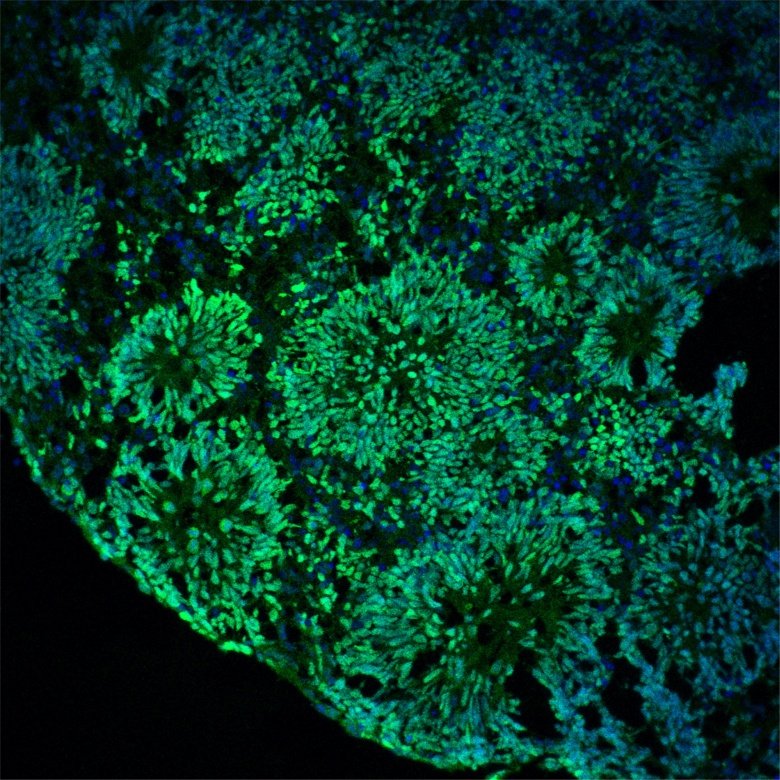
Caption:
Cerebral organoids displaying three-dimensional ventricle organization.
Photo: Anthi C. KrontiraMental illnesses, including anxiety and depression, represent a major burden on both individuals and public health systems. This prevalence has been increasing disproportionately in women of reproductive age given increasing stressorsfrom our modern society. Exposure to stress during early life, including the prenatal period via the mother’s mental state, is among the most common risk predictors of child mental illness later in life. Many behavioral dysfunctions and long-term vulnerabilities in children are associated with maternal prenatal exposures like stress hormones or pharmacologicaltreatment for associated mental illnesses. Unfortunately, we have very little knowledge of the molecular and cellular mechanisms that mediate the transmission of these environmental agents from mother to baby and how these lead to negative mental health outcomes in the next generation.
Our research program seeks to characterize how the prenatal environment impacts human brain development, ultimatelyshaping mental health outcomes. We study human-specific exposures in complex model systems of the developing human brain (cerebral organoids), and investigate mechanisms of cell- and tissue-specific responses using state-of-art molecular and cellular biology technologies. With this work we hope to improve understanding of prenatal exposures and inform treatment decisions for stress-related disorders among pregnant women with evidence about the likely outcomes in their developing children. This is especially important during the unique prenatal period when vulnerability is high and the impact deals with two lives.
Publications
Selected publications
- Article: NEUROBIOLOGY OF STRESS. 2022;21:100496Gerstner N; Krontira AC; Cruceanu C; Roeh S; Puetz B; Sauer S; Rex-Haffner M; Schmidt MV; Binder EB; Knauer-Arloth J
- Article: INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES. 2022;23(19):11448Czamara D; Cruceanu C; Lahti-Pulkkinen M; Dieckmann L; Koedel M; Sauer S; Rex-Haffner M; Sammallahti S; Kajantie E; Laivuori H; Lahti J; Raikkonen K; Binder EB
- Article: AMERICAN JOURNAL OF PSYCHIATRY. 2022;179(5):375-387Cruceanu C; Dony L; Krontira AC; Fischer DS; Roeh S; Di Giaimo R; Kyrousi C; Kaspar L; Arloth J; Czamara D; Gerstner N; Martinelli S; Wehner S; Breen MS; Koedel M; Sauer S; Sportelli V; Rex-Haffner M; Cappello S; Theis FJ; Binder EB
- Article: CELLULAR AND MOLECULAR LIFE SCIENCES. 2022;79(2):115Dieckmann L; Cruceanu C; Lahti-Pulkkinen M; Lahti J; Kvist T; Laivuori H; Sammallahti S; Villa PM; Suomalainen-Koenig S; Rancourt RC; Plagemann A; Henrich W; Eriksson JG; Kajantie E; Entringer S; Braun T; Raikkonen K; Binder EB; Czamara D
- Article: NATURE COMMUNICATIONS. 2021;12(1):6625Schmid KT; Hoellbacher B; Cruceanu C; Boettcher A; Lickert H; Binder EB; Theis FJ; Heinig M
- Editorial comment: JOURNAL OF NEUROSCIENCE. 2020;40(23):4436-4438Krontira AC; Cruceanu C
- Review: BIOLOGICAL PSYCHIATRY. 2019;86(6):433-442Elbau IG; Cruceanu C; Binder EB
- Article: MOLECULAR PSYCHIATRY. 2018;23(10):2050-2056Cruceanu C; Schmouth J-F; Torres-Platas SG; Lopez JP; Ambalavanan A; Darcq E; Gross F; Breton B; Spiegelman D; Rochefort D; Hince P; Petite JM; Gauthier J; Lafreniere RG; Dion PA; Greenwood CM; Kieffer BL; Alda M; Turecki G; Rouleau GA
- Letter: MOLECULAR PSYCHIATRY. 2017;22(9):1228-1229Cruceanu C; Lopez JP; Tsai W-T; Turecki G
- Editorial comment: JOURNAL OF NEUROSCIENCE. 2017;37(16):4225-4227Matosin N; Cruceanu C
- Article: AMERICAN JOURNAL OF PSYCHIATRY. 2015;172(11):1131-1140Cruceanu C; Tan PPC; Rogic S; Lopez JP; Torres-Platas SG; Gigek CO; Alda M; Rouleau GA; Pavlidis P; Turecki G
- Article: BRAIN BEHAVIOR AND IMMUNITY. 2014;42:50-59Torres-Platas SG; Cruceanu C; Chen GG; Turecki G; Mechawar N
Funding
Current funding
- Karolinska Institutet Faculty Funded Assistant Professorship and starting grant
- The Strategic Research Area Neuroscience (StratNeuro) Starting grant
- Strategic Recruitment Grant from Karolinska Committee for Research
- Sven and Ebba-Christina Hagberg Prize
Past Funding
- Canadian Institutes of Health Research Postdoctoral Fellowship
- Banting Postdoctoral Fellowship, Canadian Institutes of Health Research
- Individual Fellowship, Marie-Sklodowska-Curie Action
- Postdoctoral Research Fellowship, Alexander von Humboldt Foundation
- Doctoral Training Award, Canadian Institutes of Health Research
- Doctoral Training Award, Fonds de Recherche en Santé du Québec
- Gérard-Bouchard Scholarship, Réseau de médecine génétique appliquée
- F.S.B. Miller Doctoral Fellowship, Faculty of Medicine McGill University
- Alexander McFee Master’s Fellowship, Faculty of Medicine McGill University
- Society for Biological Psychiatry
- Boehringer Ingelheim
- Gairdner Foundation & Genome Canada
- Wellcome Trust Advanced Courses
Staff and contact
Group leader
- Cristiana CruceanuAssistant Professor
All members of the group
- Julia BeckmannResearch Assistant
- Cristiana CruceanuAssistant Professor
- Ilknur Safak DemirelPhd Student
- Malva DoroshenkoResearch Assistant
- Tim SchäferResearch Assistant
Work with us!
We are looking for motivated and creative individuals who are curious about human neurodevelopment, environmental exposure and psychiatric risk. Experience in microscopy, in vitro cell culture systems, single-cell and bulk transcriptomics and epigenomics, and bioinformatics is highly applicable. For more information about ongoing projects please see our research.
If you are interested in our research and would like to join the lab at the MSc, PhD or postdoc level, please send an email to: cristiana.cruceanu@ki.se.
Collaborations
We currently collaborate with research groups in Australia, Canada, Finland, Germany, Iceland, Sweden and the United States. We are always open to establishing new collaborations. Please reach out at: cristiana.cruceanu@ki.se.
Projects
Image:
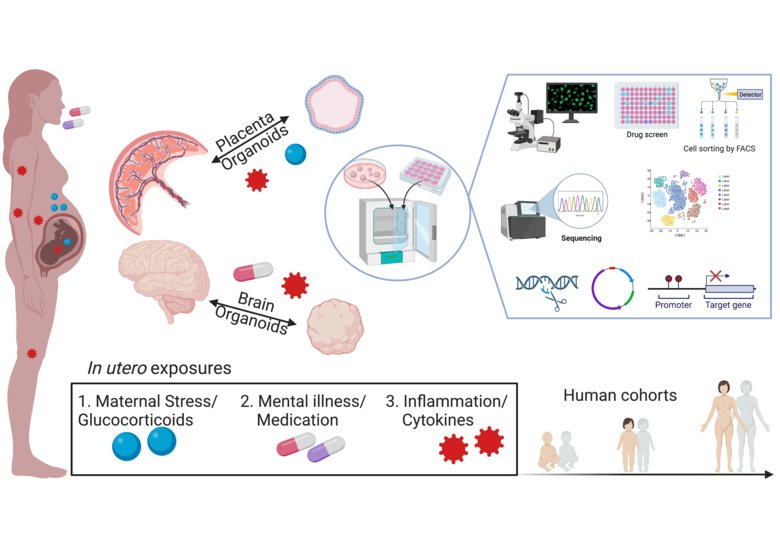
Caption:
Cruceanu Lab research program overview: Effects of exposure to stress hormones (blue), inflammation (red) and psychotropic treatment (pink/white/purple) in the mother are modelled in cerebral and placenta organoids to study the potential effects on child prenatal neurodevelopment.
Photo: Illustrator Cristiana Cruceanu, created with BioRender.Our ongoing projects are organized in three research areas.
Research Area 1: Placental mediation of in utero Glucocorticoids transmission
While the importance of stress hormones (glucocorticoids) in neurodevelopment is clear, critical questions remain regarding maternal-to-fetal glucocorticoid transmission during prenatal stress, consequently leading to aberrant hormone levels in the developing brain. We aim to characterize the molecular risk factors for placental barrier aberrant function, and hypothesize that an ineffective barrier could explain the relationship between maternal depression and anxiety and negative child outcomes.
Research Area 2: Impact of psychotropic medications on prenatal brain development
During the prenatal period, maternal mental illness and associated treatment represent some of the primary environmental factors associated with fetal brain development outcomes. Despite the prevalence of these exposures, the molecular and cellular mechanisms leading to fetal outcomes constitutes a major gap in our knowledge. Our research projects aim to elucidate (1) how/if different psychotropic medications have lasting effects on brain development, potentially shaping behavioral or cognitive outcomes later in life and (2) where is the risk/benefit balance between ongoing maternal mental illness – thus chronic prenatal stress exposure – and different treatments.
Research Area 3: Maternal prenatal inflammation and negative outcomes on offspring brain development
Inflammation and immune response, including during pregnancy, is strongly linked to glucocorticoid metabolism, and consequently stress and mental illness – thus constituting a major area of potential fetal environmental exposure. The aim of this line of research is to understand (1) how maternal inflammation agents like cytokines are transmitted to the fetus directly or (2) how they orchestrate a potential fetal inflammatory response, ultimately leading to neurodevelopmental and cognitive outcomes.
NeSTED
The Working Group of Neuropharmacology and Stress/Trauma Exposure during Development
Research and activities
May 2023: the Cruceanu and Matosin groups receive a Collaborative Research Grant from the International Brain Research Organization (IBRO).
Meet our core members
Image:

Caption:
Cristiana Cruceanu
Photo: Juan Pablo LopezDr. Cristiana Cruceanu
Assistant Professor, Department of Physiology and Pharmacology
Karolinska Institutet, Stockholm, Sweden
Role in NeSTED: In vitro models, exposure during prenatal periodsMeet our core members
The Cruceanu laboratory aims to elucidate molecular mechanisms of how the prenatal environment is transmitted to the fetus and impacts human brain development, potentially shaping life-long mental health and illness. To study human-specific processes in difficult to access developing tissues, we use complex in-vitro model systems of the developing human brain and placenta (organoids) as well as primary tissues. The lab focuses on understanding exposures related to stress metabolism and pharmacological interventions like antidepressant treatment, and their impact on neurodevelopment and early embedding of mental illness risk or resilience.
Image:

Caption:
Professor Thorhildur Halldorsdottir
Photo: Ágústa Sigurlaug GuðjónsdóttirDr. Thorhildur Halldorsdottir
Assistant Professor, Department of Psychology
Reykjavik University, Reykjavik, Iceland
Role in NeSTED: Human cohorts and health registries, stress exposure during childhood and adolescence, gene-by-environment interactions
The Halldorsdottir Laboratory aims to uncover why some youth develop psychiatric symptoms following early life stress exposure while others do not. Specifically, our goal is to understand how stressful and traumatic experiences in childhood or adolescence alter developmental and genetic processes in ways that increase an individual's risk for psychopathology. We use multidisciplinary methods drawn from clinical and developmental psychology, behavioral genetics and psychiatric epidemiology to bring us closer to this goal.
Image:

Caption:
Juan Pablo Lopez.
Photo: Cristiana CruceanuDr. Juan Pablo Lopez
Assistant Professor, Department of Neuroscience
Karolinska Institutet, Stockholm, Sweden
Role in NeSTED: Animal models of stress and treatment response.
As part of NeSTED, the Lopez Laboratory aims to identify, characterize, and manipulate cell populations and circuits that underlie susceptibility and resilience to stress-related psychiatric disorders. Our experiments are performed in male and female mice, during early life and adolescence, which are critical developmental periods associated with the etiology of psychiatric disorders. In addition, we aim to investigate if exposure to antidepressants during pregnancy or early life can affect normal development.
Image:

Caption:
Natalie Matosin, Senior Research Fellow (Assistant Professor), Molecular Horizons Institute, University of Wollongong, Wollongong, Australia.
Photo: N/ADr. Natalie Matosin
Senior Research Fellow (Assistant Professor), Molecular Horizons Institute
University of Wollongong, Wollongong, Australia
Role in NeSTED: Human brain, early life stress.
The Matosin Lab provides a crucial puzzle piece to NeSTED, specifically by contributing human brain samples, both postmortem and ex vivo. This bridges the in vivo, in vitro, and clinical cohort studies from the other group members. Using human brain tissues, we specifically focus on understanding the persistent cellular and molecular pathology that originates from early life stress directly in the human brain, which is crucial for both understanding the aetiology of stress-induced psychopathology as well as validating putative treatment targets for drug development.
Image:

Caption:
Janine Arloth
Photo: N/ADr. Janine Knauer-Arloth
Research Group Leader, Medical Genomics Group, Max Planck Institute of Psychiatry, Department of Genes and Environment, Munich, Germany; and Helmholtz Munich, Computational Health Center, Neuherberg, Germany.
Role in NeSTED: Integration of psychiatric genomics and AI to elucidate the effects of adult stress and inflammation on psychiatric disorders.
As a member of NeSTED, the Knauer-Arloth Lab integrates psychiatric genomics with AI-driven research to examine genetic and epigenetic factors that contribute to psychiatric conditions. Using advanced bioinformatics and AI methods, our team analyzes large-scale clinical and multimodal datasets, focusing on the interplay between genetic and epigenetic aspects of stress responses, their interactions with the immune system, and their long-term impact on mental health. Our expertise in modeling regulatory gene networks and dissecting subgroups is crucial in identifying mechanisms associated with disease, thereby deepening our understanding of stress-related psychiatric vulnerabilities. Our primary goal is to elucidate the underlying processes of psychiatric disorders and to translate our findings into improved diagnostic and treatment strategies, effectively bridging the gap between scientific research and clinical practice in psychiatry.