Research theme: Cancer

The thioredoxin system and cancer
It is well established that cancer cells generate ROS at higher rates than normal cells as a result of oncogenic signaling, distorted metabolism, impaired mitochondrial function and other mechanisms. Their antioxidant systems are thereby pushed to their limits, which renders tumor cells more vulnerable than normal cells to treatments that either enhance the oxidative stress or lower the antioxidant capacity. This fact may be utilized to selectively kill tumor cells. Indeed, many classical anticancer treatments - at least in part – are today known to act by increasing the ROS levels in cancer cells. Metabolic perturbations in cancer cells that increase their oxidative stress also activate the major antioxidant Nrf2-driven Trx and GSH systems. The Trx system promotes cellular growth, viability and replication through antioxidant and antiapoptotic functions, and by synthesis of DNA precursors through ribonucleotide reductase. Several electrophilic anticancer drugs target the highly reactive Sec residue of TrxR1, which can explain parts of their cytotoxicity. In order to fully understand the properties of TrxR1 as a molecular target for anticancer therapy, we need to understand the full molecular details of the functions of this enzyme. We have also found that selenium compromised forms of TrxR1 can induce cell death by a gain of function. That effect may be of importance for induction of cell death upon treatment with certain specific electrophilic agents. We named those TrxR-derived proteins SecTRAPs (Selenium compromised thioredoxin reductase-derived apoptotic proteins) and the formation of SecTRAPs may contribute to efficacy or side effects of certain electrophilic drugs used in cancer therapy, such as cisplatin (see more on SecTRAPs below). In addition, we and others have shown that the same compounds that target TrxR1, or the genetic absence of TrxR1 in conditional knockout mouse models, will induce robust Nrf2 responses in normal cells; that, in turn, may potentially augment antitumoral immunity. Overall, our studies on the Trx system in cancer collectively aim at better understanding the properties of TrxR1 and its downstream substrates, in order to probe their features and roles as target molecules for anticancer therapy with anticancer drugs targeting TrxR1. Further reading in "The thioredoxin system in cancer", ”Thioredoxin Reductase Inhibition for Cancer Therapy” and “Targeting the Selenoprotein Thioredoxin Reductase 1 for Anticancer Therapy”.
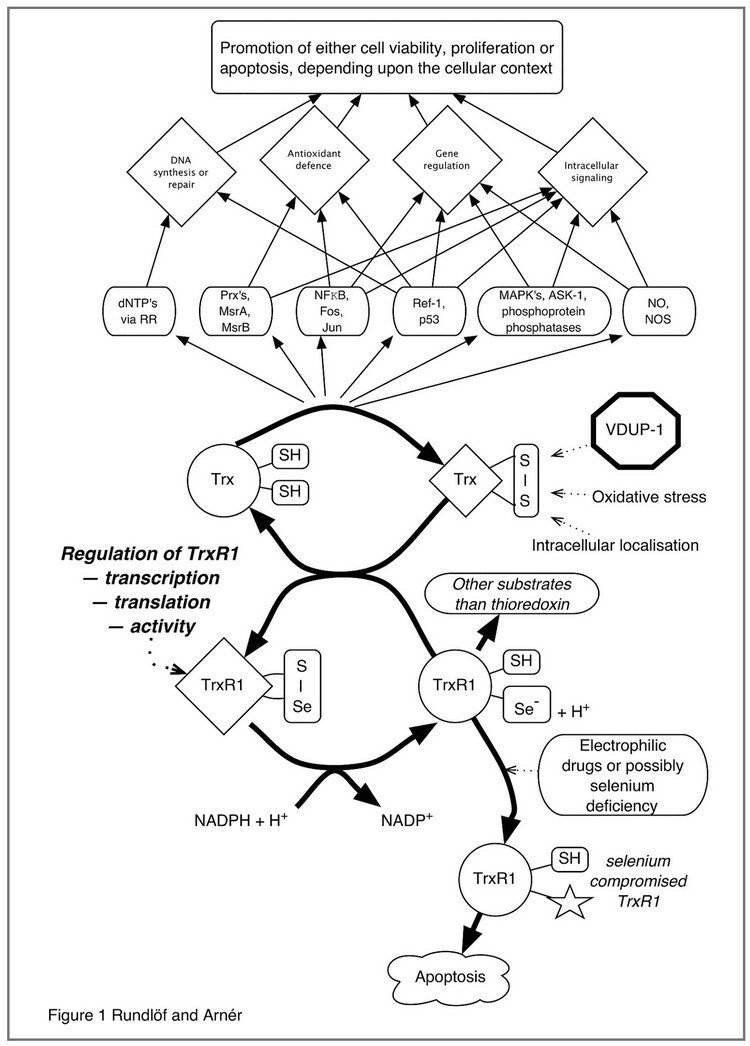
This figure illustrates the complex interplay of thioredoxin-regulated cellular functions linked to DNA synthesis, Antioxidant defense, Gene regulation and Intracellular signaling pathways that are modulated by or dependent upon reduction by thioredoxin. The redox state of thioredoxin is, in turn, dependent upon the activity of thioredoxin reductase, as well as other factors such as VDUP-1 (TBP2), oxidative stress as well as intra- or extracellular localization. It should be noted that most, if not all, of the functions of the thioredoxin system are dependent upon thioredoxin reductase(s), which are selenium dependent enzymes also reducing a number of other substrates in addition to thioredoxin. Targeting of thioredoxin reductase is also a potentially important mechanism for anticancer efficacy of electrophilic drugs.
Figure taken from "Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events".
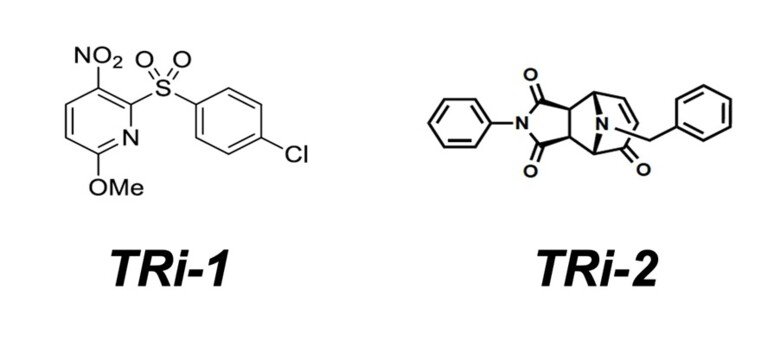
TRi-1 – a new and more specific TrxR1 inhibitor
We performed a high-throughput screen together with NCATS NIH, USA, for discovery of novel and more specific inhibitors of TrxR1. The screen results were made public through PubChem (Assay ID: 588453) and the initial results were published in 2018 (see “Irreversible inhibition of cytosolic thioredoxin reductase 1 as a mechanistic basis for anticancer therapy”). To briefly summarize the study, we tested close to 390,000 compounds, each with 6-fold dilution curves, and using preset criteria we identified ≈3500 small molecule TrxR1 inhibitors. Cherry-picking for novelty, selectivity, solubility indices, and “druggability” we narrowed down the list to 54 compounds that were further tested. We assessed these compounds in cytotoxicity screens with correlations to cellular redox systems, effects on TrxR1, correlations with anticancer efficacy, and for the top hits we performed in vivo cytotoxicity assays using different mouse models, with or without human xenografts or syngenic mouse tumors. The results with the top hits were highly promising. We did not see any overt toxicity to healthy tissues of mice but obtained clear anticancer efficacy with the two top compounds, called TRi-1 and TRi-2 (thioredoxin reductase inhibitor 1 and 2). Using SAR analyses we identified active properties of the molecules and, importantly, achieved specificity in cytosolic TrxR1 targeting with low inhibition of mitochondrial TrxR2 (not obtained previously), and could thus solidify the notion of a TrxR1-dependent anticancer efficacy. Later follow-up studies with comprehensive proteomics as well as the new RX1 probe (see our page on Biotechnology) have confirmed that TRi-1 is the most specific TrxR1 inhibitor yet described. See ”Comprehensive chemical proteomics analyses reveal that the new TRi-1 and TRi-2 compounds are more specific thioredoxin reductase 1 inhibitors than auranofin” and “Selective cellular probes for mammalian thioredoxin reductase TrxR1: rational design of RX1, a modular 1,2-thiaselenane redox probe” for further details.
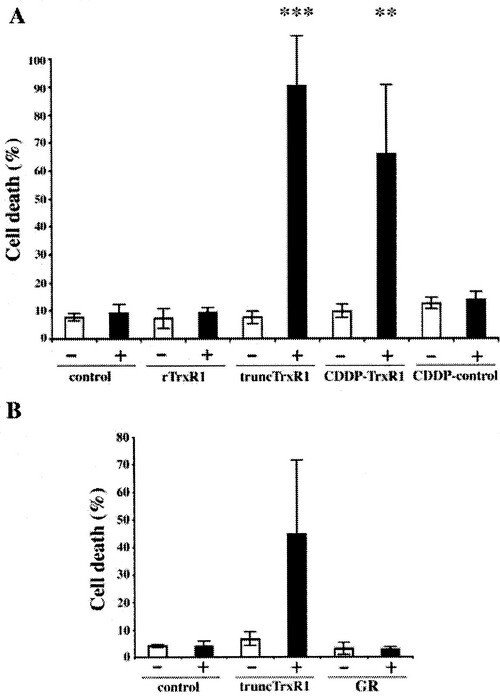
SecTRAPs
We discovered that TrxR1 either deficient in selenocysteine or with the selenocysteine residue derivatized by electrophilic compounds, such as cisplatin, could directly induce apoptosis when introduced into cells. We named these pro-oxidant forms of TrxR1, which are derivatised at the Sec residue but still able to sustain an NADPH oxidase activity, for SecTRAPs (Selenium compromised thioredoxin reductase-derived apoptotic proteins; also indicating that the Sec residue acts like a ”trap” for many electrophilic agents). Later studies have revealed that the cell death triggered by SecTRAPs is not only apoptotic, but rather a mixture of different forms of cell death that correlates with excessive oxidative stress.This phenomenon may explain some of the mechanism for therapeutic effects of electrophilic drugs used in cancer therapy and potentially also some of the symptoms seen in selenium deficiency.
The first report was described in the following paper "Rapid induction of cell death by selenium-compromised thioredoxin reductase 1 but not by the fully active enzyme containing selenocysteine".
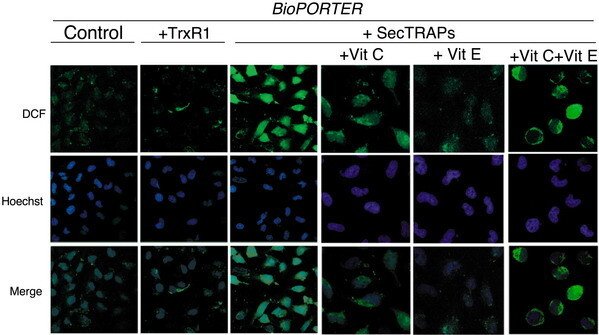
HeLa cells were treated with BioPORTER, TrxR1/BioPORTER or SecTRAPs/BioPORTER for 3 h as indicated in the figure, whereupon the images of the cells stained with the ROS-sensitive marker DCFH were taken, as described in the text. The cell nuclei were counterstained with Hoechst 33342. Ascorbic acid (Vit C) and/or ŚÁ-tocopherol (Vit E) was added 1 h in advance to the cells before treatment. Two distinct experiments with two samples in each treatment were performed with similar results and representative images of the observed staining patterns are shown. The three right-most pictures displaying the intracellular patterns of DCF fluorescence are shown at higher magnification than the three panels to the left displaying the overview of DCF fluorescence in control cells or in cells treated with either TrxR1 or SecTRAPs together with BioPORTER.
This result was described in "Cell death by SecTRAPs: thioredoxin reductase as a prooxidant killer of cells".
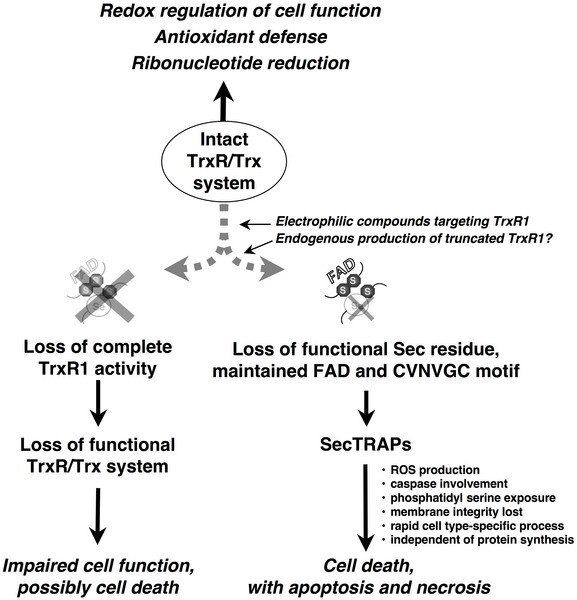
In the same paper, we have also concluded the following model for the formation as well as the function of the SecTRAP.
We propose that during most conditions of normal cell growth the Sec residue in TrxR1 is intact and the role of this enzyme is thus to sustain the many cellular functions of the thioredoxin system. This activity is dependent upon the redox active C-terminal Sec-containing active site in TrxR1. SecTRAPs may however be formed from TrxR1 if its Sec residue becomes compromised, either by removal or by derivatization with electrophilic compounds, while the FAD and N-terminal redox active CVNVGC motif of the enzyme are kept intact.
SecTRAPs lack the Trx reducing activity of native TrxR1 but become potent inducers of cell death by a gain of function. This cell death is, as shown in the present study, rapid, does not require induction of protein synthesis, involves production of reactive oxygen species and is prevented by caspase inhibitors. If, on the other hand, both the CVNVGC motif and the C-terminal selenolthiol motif become inactivated, e.g. by certain types of TrxR1 inhibitors or if the overall expression of TrxR1 is diminished in cells, impaired cell function or cell death may still occur. In such cases the cellular consequences would however not be due to a gain of function in derivatives of TrxR1, but rather to hampered functions of the complete cellular thioredoxin system.
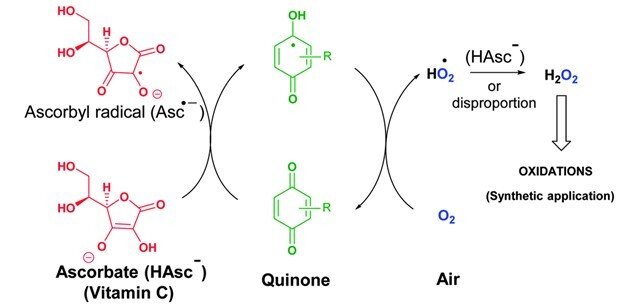
The combination of vitamin C and vitamin K3 in cancer treatment
Cancer cells have diverse genetic backgrounds but they share common characteristics, such as a higher level of ROS and addiction to glycolysis (Warburg effect). The combination of ascorbate and menadione (VC/VK3) targets both features to selectively kill cancer cells. VC/VK3 forms a redox cycle to generate H2O2, inducing oxidative stress. Due to its similar structure to glucose, the oxidized VC, dehydroascorbic acid (DHA), can enter cancer cells more easily via the overexpressed glucose transporters (GLUTs). We found that VC/VK3 disrupted the major antioxidant systems in cancer cells, leading to oxidation of Trx and GSH systems that are essential for the supply of electrons to ribonucleotide reductase (RNR), the only enzyme catalyzing the de novo synthesis of deoxyribonucleotides (dNTP) for DNA replication and repair. The inadequate dNTP production cause replicative stress in cancer cells. The results were published in 2019 in “The combination of ascorbate and menadione causes cancer cell death by oxidative stress and replicative stress”.
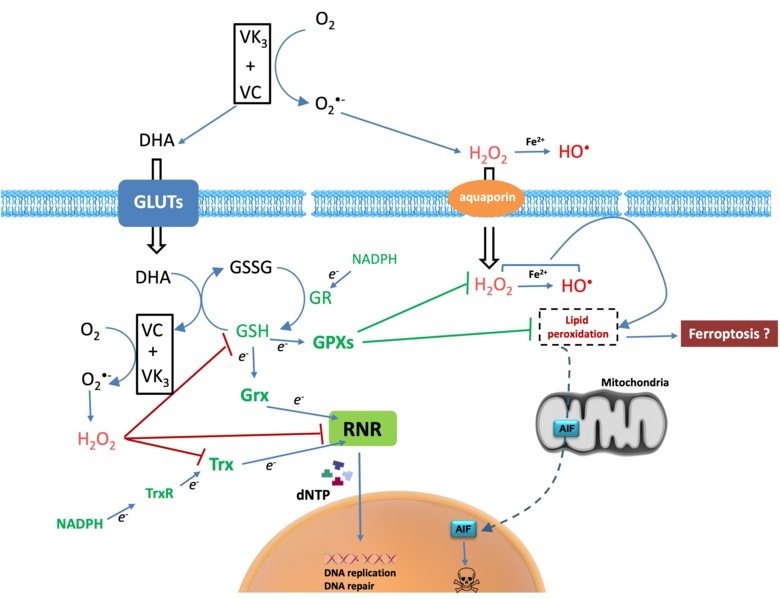
We are also investigating the mechanism of cell death induced by the treatment and searching for potential synergistic compounds to overcome chemoresistance. Clinical trials are underway to evaluate the safety and efficacy of the combination of VC/VK3 (Apatone®) as an anticancer treatment.
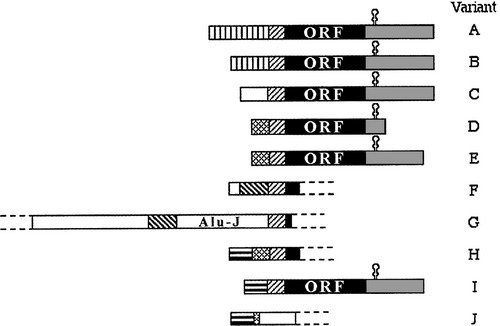
Splice variants of TrxR1 protein isoforms, different 5'-UTR cDNA's and TXNRD1 variants
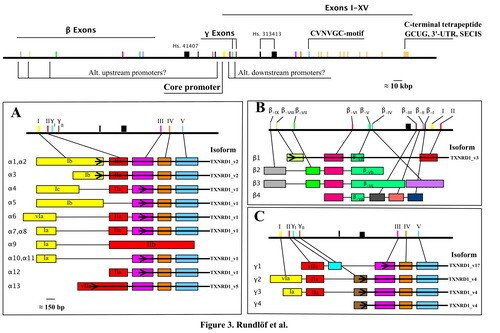
We have found evidence of a number of different splice variants of TXNRD1-derived transcripts, resulting in both different TrxR1 protein isoforms and a number ofl cDNA variants encoding the same protein product. The significance of these variants is not known and they are currently further studied in our laboratory.
Our initial findings are presented and further discussed in "Prominent expression of the selenoprotein thioredoxin reductase in the medullary rays of the rat kidney and thioredoxin reductase mRNA variants differing at the 5' untranslated region" and "Evidence for intriguingly complex transcription of human thioredoxin reductase 1".
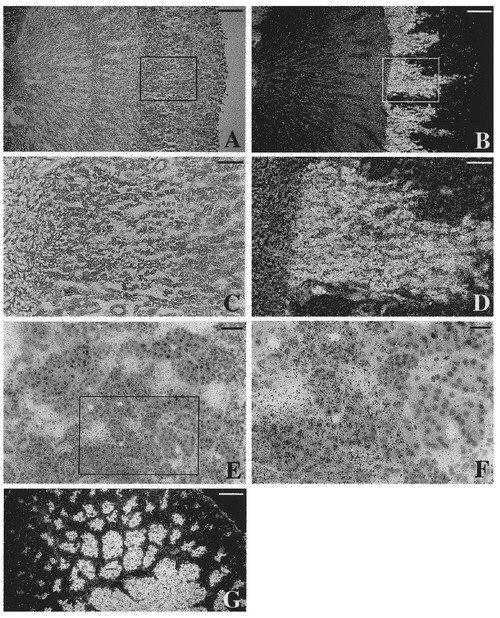
In the same study where the different murine 5'-UTR domains were first found and discussed (previous figure), in situ hybridization using adult rat kidney was also performed, inspired by surprisingly high levels of TrxR1 mRNA in rat kidney as judged by Northern blots. The expression pattern was highly organized, as seen in this figure, with a prominent expression in the medullary rays. The pattern of expression exactly follows the proximal tubules, which is interesting as these cells are known to produce another selenoprotein, plasma glutathione peroxidase, which is secreted to plasma from these cells.
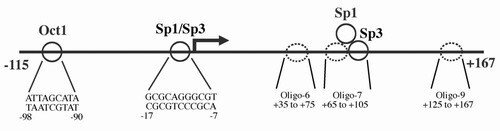
We identified the core promoter for human TrxR1 and found it to have typical characteristics of a housekeeping gene, which has interest in view of the mRNA being regulated by AU-rich elements. Further reading in "The core promoter of human thioredoxin reductase 1: cloning, transcriptional activity, and Oct-1, Sp1, and Sp3 binding reveal a housekeeping-type promoter for the AU-rich element-regulated gene".
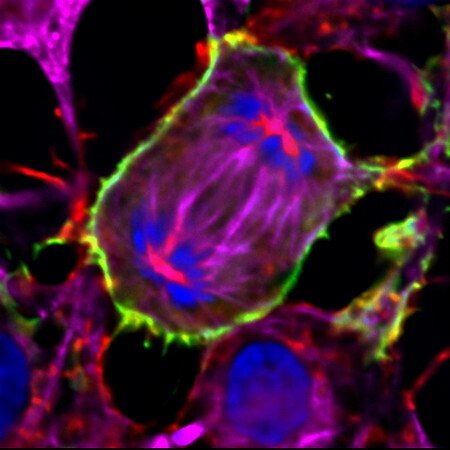
TXNRD1_v3's unique expression pattern and it's role in the cell membrane protrusions
The TXNRD1_v3 isoform of human TrxR1 (short form notation "v3") has an atypical glutaredoxin domain in fusion with the common TrxR1 core module. It is encoded by the β1 transcript, which is transcribed from a promoter upstream of the more commonly used core promoter. We found that v3 is highly expressed in Leydig cells of the testis, but it is also found in ovary, some additional tissues and a number of cancer cell lines, it is responsive to sex hormone treatment, and it localizes to cell membranes in transfected cells. The glutaredoxin domain of this protein was also found to have the capacity to induce a formation of cell membrane protrusions, with actin and tubulin polymerisation following v3 into the protrusions. The biological importance of v3 is still unknown.
This was a collaborative project with Anastasios Damdimopoulos, Antonio Miranda-Vizuete and Alberto Jimenez.
Figure taken from "Induction of cell membrane protrusions by the N-terminal glutaredoxin domain of a rare splice variant of human thioredoxin reductase 1".
